Abstract
Myocardial perfusion imaging (MPI) provides additional clinical information on children with cardiac disease but will not benefit children with chest pain and normal cardiac studies. This study reviewed all technetium-99 m (99mTc) sestamibi stress MPI studies between 2004 and 2010 performed in association with graded exercise testing (86% with bicycle ergometer, 14% with treadmill). A positive test was defined as a perfusion defect or abnormal ventricular function response. Clinical records were reviewed, including follow-up assessment to determine accuracy of MPI interpretation. False-positive and false-negative rates were recorded. A total of 197 patients (mean age, 13.4 ± 3.6 years, 70% male) underwent 218 MPI studies. Group A had 42 patients (43 studies) with isolated chest pain and normal studies. Of the 43 studies, 39 had negative results, and 4 had false-positive results. Group B had 155 patients (175 studies) with known or suspected cardiac disease, and 39 tests (33 patients) had positive results. Whereas 32 studies were considered true-positive, 7 were false-positive. There was one false-negative test. According to the findings, 99mTc sestamibi MPI studies are clinically useful but not perfect tests in the setting of known or suspected cardiac disease based on clinical evaluation, electrocardiography (ECG), or echocardiography. Children who had isolated chest pain with a normal ECG and echocardiogram often have false-positive studies.
Similar content being viewed by others
Myocardial perfusion imaging (MPI) is beneficial for detecting ischemic heart disease in patients (usually adults) with coronary artery disease. Most cardiac disease causing chest pain and ischemia in children may be detected by history, physical examination, electrocardiography (ECG), and echocardiography (echo). These causes include anomalous origin of the coronary arteries, coronary artery aneurysms from Kawasaki disease, congenital heart disease, and hypertrophic cardiomyopathy. However, for children who have chest pain without these conditions, the etiology is highly unlikely to be cardiac [12]. We hypothesized that children who have suspected cardiac ischemic disease with positive ECG or echo findings would benefit from MPI, whereas those who have chest pain with normal studies (i.e., normal ECG and/or normal echo) would not benefit.
Methods
We obtained institutional review board approval from our institution to perform a retrospective review of all technetium-99m (99mTc) sestamibi perfusion imaging studies between January 2004 and November 2010. Referral was for nuclear perfusion imaging to be performed in association with graded exercise testing. The indication for myocardial perfusion study was probable or possible impairment of myocardial perfusion. A total of 197 patients (mean age, 13.4 ± 3.6 years, 70% male) underwent 218 perfusion imaging studies.
Cohort Demographics
The chest pain group (group A) had 42 patients (43 studies) with isolated chest pain and normal ECG and echo (3 patients did not have an echo). Chest pain was described as exertional for 32 patients (33 tests). The group A cohort is described in Table 1.
The cardiac diagnosis group (group B) had 155 patients (175 studies) with known or suspected cardiac disease. The group B cohort is described in Table 2. The 155 group B patients included 101 patients with known congenital or acquired heart disease as well as 54 patients for whom congenital or acquired heart disease was suspected clinically by history or lab data but not confirmed. A complete list of the cardiac diagnoses in group B is provided in Appendix A as ESM. All the patients had complete review of their records for relevant cardiac diagnostic studies.
Exercise Tests
The majority of the studies (86%, 188/218) were performed using a previously calibrated, electronically braked cycle ergometer (Sensormedics VIA Sprint 150P, Yorba Linda, CA, USA). The pedaling rate was maintained at 75–85 rpm. All the subjects exercised to exhaustion. The subjects were monitored for cardiac arrhythmia during the exercise and 10 min afterward.
The workload was based on the patient’s weight and sex. Males underwent a workload of 0.33 W/kg/min rounded to the nearest 5 W. Females underwent a workload of 0.30 W/kg/min rounded to the nearest 5 W. The initial workload was zero, which increased at 10, 15, 20, or 25 W/min depending on the aforementioned formula. Of the 218 studies, 30 (14%) were performed using the treadmill (Series 2000 Treadmill; GE Marquette Medical Systems, Milwaukee, WI, USA) according to a Bruce protocol [2].
The data measured were weight, height, resting and peak heart rate, respiratory rate, blood pressure, tidal volume, resting/peak oxygen consumption (VO2), exercise time, and total work. Respiratory gases were collected by mouthpiece and analyzed by a metabolic cart (Vmax Encore 29C; Sensormedics, Yorba Linda, CA, USA). Oxygen consumption was measured on a breath-by-breath basis.
During the exercise test, heart rate, 12-lead ECG, and blood pressure were monitored. Indirect blood pressure was measured using a blood pressure cuff with audio amplification (Tango Stress Blood Pressure machine; SunTech Medical, Morrisville, NC, USA). The first and fourth Korotkoff sounds were used for systolic and diastolic blood pressures.
Resting and peak exercise 99mTc sestamibi injection with perfusion imaging was performed using a gamma camera (ECAM; Siemens, Hoffman Estates, IL, USA). The resting study was performed first followed by the stress portion 2 h later. At 1 min before peak exercise, the stress dose of sestamibi was injected intravenously. The patient exercised for at least 1 min longer after the injection.
Before 10 October 2008, the resting dose of 99mTc sestamibi was 200 μCi/kg, with a minimum of 4 mCi and a maximum of 15 mCi. The stress dose was 400 μCi/kg, with a minimum of 8 mCi and a maximum of 30 mCi. After 10 October 2008, the resting dose was 150 μCi/kg, with a minimum of 2 mCi and a maximum of 10 mCi. The stress dose was 450 μCi/kg, with a minimum of 6 mCi and a maximum of 30 mCi.
After the dosage change, 58 studies (27%; 58/218) were performed, with the majority (73%) conducted before the dosage change. On 28 October 2010, a third change was made that reduced the resting dose to 100 μCi/kg, with a minimum of 2 mCi and a maximum of 10 mCi. The stress dose was reduced to 300 uCi/kg, with a minimum of 6 mCi and a maximum of 30 mCi. This change involved four patients.
A positive test was defined as a perfusion defect or an abnormal left ventricular response to exercise (decreased function). We did not count right ventricular hypertrophy, left ventricular hypertrophy, or paradoxical septal motion as positive.
Follow-up tests to confirm coronary perfusion abnormalities included cardiac magnetic resonance imaging (MRI; some with delayed enhancement), computed tomography (CT), angiogram, cardiac catheterization, and, in some cases, a repeat myocardial perfusion test.
Data Analysis
Descriptive statistics were collected. Positive predictive value was calculated using standard epidemiologic practices when relevant.
Results
Of the 43 studies (42 patients) in group A, 39 were negative. There were four false-positive tests based on later normal cardiac MRI for three patients and a repeat perfusion imaging study, which was normal for one patient (Table 3). Thus, no patients in group A had coronary artery disease.
Of the 155 studies (175 patients) in group B, 39 (33 patients) were positive. There were seven false-positive tests based on normal cardiac catheterization in six patients and repeat normal myocardial perfusion tests and CT angiography in one patient (Table 4). There was one false-negative test. The details are provided later.
Congenital Heart Disease
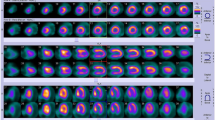
The patients with transposition of great arteries (TGA) after an arterial switch operation (ASO) were the most frequent patients with heart surgery sent for nuclear perfusion study (n = 15). There was one true-positive test, for an asymptomatic patient with myocardial perfusion imaging that showed ST-T changes in leads V4-6 together with reversible ischemia of the apex and anteroseptal wall. Left anterior descending coronary artery occlusion was confirmed at cardiac catheterization (Fig. 1).
Patient B-28 (January 2010) with transposition of the great arteries after an arterial switch operation. Age at study was 9.5 years. a Vertical view of the left ventricle at rest with a sestamibi injection. b The same view after stress test. Note the decreased perfusion (more purple) near the apex of the left ventricle at stress representing ischemia. c Electrocardiogram representing V5 at rest. d Electrocardiogram at peak stress showing ST-T depression representing ischemia in V5 (laterally). Cardiac catheterization shows total occlusion of the left anterior descending coronary artery. The patient went on to have surgery to correct this
Three patients with dextro-TGA (d-TGA) after ASO had false-positive studies documented by findings at cardiac catheterization or CT angiogram (Table 4). One false-negative study occurred in this group (patient B-131). For this patient, the cardiac catheterization showed paradoxical interventricular septum motion and left coronary artery stenosis, so the MPI test was a false-negative. A second patient (B-151) presented with a cardiac arrest and ventricular fibrillation 9 months after a normal MPI test. Cardiac catheterization showed an externally compressed left coronary artery. Thus, in this group of patients with TGA, one test unmasked unsuspected disease, but four tests were inaccurate. For one patient, the test failed to detect ischemic disease 9 months before an ischemic event (Table 5).
Six patients (7 studies) were sent with tetralogy of Fallot (TOF) repair. Four tests for three patients were positive, and three tests were negative. One patient (B-15) with positive test results had external compression of the left anterior descending coronary artery from the right ventricle (RV) to the pulmonary artery conduit, and another patient (B2) had surgery to revise the RV outflow tract due to ischemic changes and perfusion changes on the MPI (Table 6).
Four patients (6 scans) had single-ventricle physiology and had undergone Fontan operations. Five of the six studies had positive results. One patient (B24) had an RV ejection fraction (EF) decreased to 36%, with reduced uptake in the RV. The patient later died in the cardiac catheterization lab. Autopsy showed a massive myocardial infarction of the RV (Table 7). The remaining patients with positive test results were followed medically.
Other Heart Disease (Appendix B as ESM)
A total of 13 patients (15 studies) with cardiomyopathy were referred for decreased cardiac function shown on echo. One patient had false-positive test results showing a reversible anterior perfusion defect, but catheterization did not confirm a coronary abnormality. Seven patients had positive test results with decreased function (2 with probable myocarditis and 5 with cardiomyopathy of unclear etiology). None of these patients had coronary artery disease. Six patients had idiopathic hypertrophic subaortic stenosis. Three of the six patients (B-21, B-13, B-32) had positive myocardial perfusion test results, and one of the patients (B-21, 2008 asymptomatic) had an automated external defibrillator placed. Patient B-13, the remaining patient with the positive test results, was already receiving beta-blockers, and this patient’s chest pain was not thought to be cardiac. Patient B-32 was receiving beta-blockers.
A total of 17 patients (18 studies) were referred for coronary artery anomalies. All studies after anomalous origin of the left coronary artery from the pulmonary artery (ALCAPA) repair and after those with small coronary artery fistulas were negative. Six patients had right coronary artery anomalies from varying etiologies. There was one false-positive involving a patient (B-18) who presented with chest pain at rest. Echo showed that she had anomalous origin of the right coronary artery (RCA) from the left coronary artery (LCA) (intramural course), confirmed by cardiac catheterization and MRI. Postoperative MPI study showed ischemia in the anteroseptal region, which is the distribution of the LCA. The next day, cardiac catheterization showed good filling of both coronary arteries, so the study was false-positive. There was one true-positive involving a patient with a coil in the right coronary artery that drained to the RV. There were no ECG changes of ischemia, but there was dys-synergy of the septum and inferior walls.
A total of 12 patients (13 studies) with Kawasaki disease underwent testing. Four of these patients had coronary aneurysms at the time of testing, whereas two had regressed ectasia. Of the 13 ECGs, 12 were normal. One ECG showed unifocal, premature ventricular contractions from the RV outflow tract. The echocardiograms for 6 of the 13 patients were normal at the time of their presentation. The exercise tolerance test ECG showed no ischemia in any (13/13) of the studies, and 12 of the 13 studies showed normal perfusion and function. The positive test showed a fixed defect at the apex and hypokinesis of the septum. A follow-up MRI was ordered for patient B-26 with Kawasaki disease. The complete details can be found in Table 8.
Four studies investigated two patients after heart transplantation. One patient showed a fixed and reversible defect at the apex. The coronary arteries were patent, and he did not have graft vasculopathy. A follow-up MPI study in 2009 showed improved apical ischemia. The second patient had abnormal septal motion with normal perfusion. Cardiac catheterization showed no coronary artery graft vasculopathy.
Structurally Normal Heart With Abnormal Resting/Exercise Electrocardiogram or Syncope
A total of 26 patients had ECG changes (Appendix B as ESM). Among the exercise tests, 23 were negative, 2 were positive, and 1 was false-positive. Eight of the patients had previous exercise tolerance test abnormalities. Nine patients had resting ST-T wave changes, and seven had other changes. The first patient with a positive test showed premature ventricular contractions and low shortening fraction with decreased left ventricle ejection fraction (LVEF) on the perfusion test. The second patient with a positive test had sinus bradycardia with a superior axis and a right bundle branch block. Perfusion scan showed a decreased LVEF of 44%, and echo showed a dilated LV (5.9 cm) with a shortening fraction of 32%. The patient with a false-positive result first showed ischemia of the lateral wall with ST-T wave changes, but cardiac catheterization showed normal coronary arteries.
There were 14 patients with syncope/near syncope (Appendix B as ESM). The nuclear portions of the tests were negative for 13 of the 14 patients. The remaining patient (B-30) had positive MPI results. The patient had a reversible defect at the septum and wall motion abnormality and at this writing is to have a follow-up MRI to examine function and coronary artery anatomy.
Discussion
This study reports a large experience with MPI in a broad range of patients. For the patients with chest pain in the absence of cardiac disease, syncope without cardiac disease, small coronary artery fistulas, and anomalous origin of RCA from LCA, no true positive studies were obtained, and a significant number of false-positive studies prompted additional cardiac testing.
In contrast, for the patients with structural heart disease (e.g., after ASO for TGA, after TOF repair, single ventricle), coronary artery abnormalities (after ALCAPA repair), and cardiomyopathy, MPI often provided useful clinical information leading to further diagnostic studies and interventions. False-positives occurred in this group as well and one false-negative study. One patient with d-TGA after ASO presented with cardiac arrest from coronary occlusion 9 months after a normal MPI test, which shows the limitation of the test. Cardiac function abnormalities were detected in patients with cardiomyopathy and abnormal electrocardiograms.
The incidence of ischemia in the pediatric population with chest pain but no structural heart disease is negligible. A previous paper demonstrated the utility of thallium studies for patients with known structural heart disease such as coronary artery malformations, Kawasaki’s syndrome, TGA after ASO, dilated cardiomyopathy, and myocardial dysfunction after surgery for congenital heart disease [1]. However, chest pain is not listed as an indication for a thallium study. Our study reports the largest experience of MPI in pediatric patients with chest pain and confirms this recommendation.
For patients with congenital heart disease, MPI is helpful in some clinical situations. For TGA after ASO, the coronary arteries are reimplanted at the time of surgery and may be prone to kinking, abnormal vasodilation, or failure of the anastomosis to grow after reimplantation [15]. Our study showed that MPI could identify a coronary artery problem in an asymptomatic patient. However, one of our patients with d-TGA after ASO presented 9 months after a negative MPI test with cardiac arrest, and cardiac catheterization showed occlusion of the left main coronary artery.
For patients with a systemic RV with TGA after ASO or congenitally corrected TGA, prior studies have shown that MPI plays a role in the development of RV dysfunction [4, 5, 9, 10]. In our study, one of these patients died after a cardiac catheterization. Autopsy showed a massive RV infarction, suggesting a perfusion abnormality.
Myocardial perfusion imaging may be helpful for detecting coronary artery disease in selected patients. It is not uncommon that reversible or fixed perfusion defects persist even after repair of an ALCAPA [6]. Two patients (B-80 and B-121) did not exhibit perfusion defects but did show abnormal septal motion, possibly due to preoperative myocardial damage sustained due to myocardial steal before repair. Myocardial perfusion imaging is not helpful for diagnosing ischemia in patients with anomalous origin of RCA from LCA. Right ventricular wall thickness is one third that of the LV. Therefore, uptake of tracer by the RV myocardium is not sufficient to opacify during a resting study [14]. The RV may appear on the exercise portion of the test, but because it does not show on the resting portion, it is not helpful.
Echocardiograms were helpful in suspecting anomalous origin of RCA from LCA in four patients, with confirmation by MRI imaging for two patients and by CT scan for two patients. Similarly, MPI for patients with small coronary artery to pulmonary artery fistulas is not helpful. Sherwood et al. [13] followed 31 patients (age, 7.2 ± 8.4 years) clinically with small fistulas. During a follow-up period of 9.3 ± 9.1 years, all were asymptomatic, without a continuous murmur or adverse clinical outcome, such as ischemia. This is consistent with our study of five patients with small coronary artery fistulas, all of whom had negative MPI results.
Studies have postulated that ischemia is the mechanism in sudden death among children with hypertrophic cardiomyopathy. Dilsizian et al. [3] studied 23 patients with hypertrophic cardiomyopathy (ages 6–23 years) who had experienced previous cardiac arrest or syncope (n = 15) or a family history of sudden cardiac death (n = 8). All 15 patients with either cardiac arrest or syncope had inducible ischemia by MPI compared with only 3 of 8 patients (37%) who had no such history. Our three patients with positive MPI tests were treated with beta-blockers or placement of an implantable cardioverter-defibrillator (ICD). Thus, we found MPI useful in this population.
From a resource standpoint, there is a worldwide shortage of the isotope technetium because the National Research Universal reactor (Ontario, CA, USA) has been shut down for repairs [16]. From a radiation standpoint, the exposure to children is significant. The exposure from a regular posteroanterior chest radiograph is 0.13 mSv. The estimated resting dose from a 99mTc sestamibi resting test for a 1-year-old is 5.4 mSv or the equivalent of 415 chest radiographs. The estimated resting dose for a 5-year-old is 4.8 mSv or the equivalent of 369 chest radiographs. We can alleviate unnecessary radiation exposure by eliminating unnecessary tests [8].
Study Limitations
One limitation of this study was that the radiologists who read the report were not blinded from the clinical data, so the clinical information may have influenced their reading. Also, over the 5-year period, different radiologists read the studies, so there may have been variability in the reading of a positive study.
A second limitation was the definition of a positive test. This study’s definition of a positive test (perfusion defect or abnormal left ventricular response) may differ from the clinician’s definition of a positive test (e.g., paradoxical septal motion, left ventricular hypertrophy).
A third limitation was the correct gold standard for confirmation of a positive study. Many of these studies were followed up by cardiac catheterization, MRI, or repeat myocardial perfusion scans, which were accepted clinically as the gold standard. Also, because this study was retrospective, many patients with positive myocardial perfusion scans did not have a follow-up cardiac catheterization, MRI, or repeat myocardial perfusion study.
Recommendations
A nuclear MPI may be recommended for children with the following:
-
TGA after ASO (coronary ischemia may be unmasked in an asymptomatic patient, but this test is NOT definitive)
-
TOF after repair in cases with coronary arteries that could be compromised by surgical conduits or patches
-
Single ventricles with poor function (as an indicator of prognosis)
-
Borderline cardiac function on echo (to help confirm decreased function)
-
ALCAPA repair (to detect residual ischemic areas and therefore help guide therapy [e.g., exercise restrictions, beta blockade])
-
Kawasaki disease with known significant coronary artery involvement [7, 11].
Additionally, MPI may be useful for patients with the following:
-
Hypertrophic cardiomyopathy (to determine their sudden death risk and to plan therapy)
-
ECG changes on previous testing (to determine whether the changes are false-positives or real ischemia)
-
Heart transplant (used as an early indicator for coronary artery vascular disease to help timing of cardiac catheterization).
Myocardial perfusion is not helpful for patients with the following:
-
Chest pain with a normal ECG and echo
-
Small coronary artery to ventricle fistulas
-
Anomalous origin of RCA from LCA
-
Syncope with a normal ECG and echo.
References
Björkhem G, Evander E, White T, Lundström NR (1990) Myocardial scintigraphy with 201 thallium in pediatric cardiology: a review of 52 cases. Pediatr Cardiol 11:1–7
Cumming GR, Everatt D, Hastman L (1978) Bruce treadmill test in children: normal values in a clinic population. Am J Cardiol 41:69–75
Dilsizian V, Bonow RO, Epstein SE, Fananapazir L (1993) Myocardial ischemia detected by thallium scintigraphy is frequently related to cardiac arrest and syncope in young patients with hypertrophic cardiomyopathy. J Am Coll Cardiol 22:796–804
Hornung TS, Bernard EJ, Jaeggi ET, Howman-Giles RB, Celermajer DS, Hawker RE (1998) Myocardial perfusion defects and associated systemic ventricular dysfunction in congenitally corrected transposition of the great arteries. Heart 80:322–326
Kondo C, Nakazawa M, Kusakabe K, Momma K (1996) Myocardial dysfunction and depressed fatty acid metabolism in patients with cyanotic congenital heart disease. J Nucl Cardiol 3:30–36
Kondo C (2004) Myocardial perfusion imaging in pediatric cardiology. Ann Nucl Med 18:551–561
Lim CW, Ho KT, Quek SC (2006) Exercise myocardial perfusion stress testing in children with Kawasaki disease. J Paediatr Child Health 42:419–422
Linet M, Kim KP, Rajaraman P (2009) Children’s exposure to diagnostic medical radiation and cancer risk: epidemiologic and dosimetric considerations. Pediatr Radiol 39:S4–S26
Lubiszewska B, Gosiewska E, Hoffman P, Teresińska A, Rózański J, Piotrowski W, Rydlewska-Sadowska W, Kubicka K, Ruzyłło W (2000) Myocardial perfusion and function of the systemic right ventricle in patients after atrial switch procedure for complete transposition: long-term follow-up. J Am Coll Cardiol 36:1365–1370
Millane T, Bernard EJ, Jaeggi E, Howman-Giles RB, Uren RF, Cartmill TB, Hawker RE, Celermajer DS (2000) Role of ischemia and infarction in late right ventricular dysfunction after atrial repair of transposition of the great arteries. J Am Coll Cardiol 35:1661–1668
Newburger JW, Takahashi M, Gerber MA, Gewitz MH, Tani LY, Burns JC, Shulman ST, Bolger AF, Ferrieri P, Baltimore RS, Wilson WR, Baddour LM, Levison ME, Pallasch TJ, Falace DA, Taubert KA, Committee on Rheumatic Fever, Endocarditis and Kawasaki Disease; Council on Cardiovascular Disease in the Young; American Heart Association; American Academy of Pediatrics (2004) Diagnosis, treatment and long term management of Kawasaki disease. Circulation 110:2747–2771
Selbst SM (1986) Evaluation of chest pain in children. Pediatr Rev 8:56–62
Sherwood MC, Rockenmacher S, Colan SD, Geva T (1999) Prognostic significance of clinically silent coronary artery fistulas. Am J Cardiol 83:407–411
Sty JR, Starshak RJ (1985) Thallium-201 myocardial imaging in children. J Am Coll Cardiol 5:S128–S139
Vogel M, Smallhorn JF, Gilday D, Benson LN, Ash J, Williams WG, Freedom RM (1991) Assessment of myocardial perfusion in patients after the arterial switch operation. J Nucl Med 32:237–241
Wald M (2009) Radioactive drug for medical tests is in short supply. New York Times 24 July, A11, A16
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Robinson, B., Goudie, B., Remmert, J. et al. Usefulness of Myocardial Perfusion Imaging With Exercise Testing in Children. Pediatr Cardiol 33, 1061–1068 (2012). https://doi.org/10.1007/s00246-012-0226-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00246-012-0226-7