Abstract
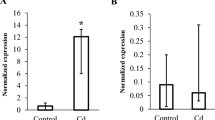
Currently, there is limited information on the toxicity of low concentration of metal mixtures in the environment. Of particular interest is the effect of low levels of metal mixtures on neurodevelopment of aquatic organisms. This study reports the neurological gene expressions after exposing zebrafish embryos to low concentration toxic heavy metals, 120 h post fertilization (hpf). Embryos were exposed to low concentration individual and mixtures of lead (Pb), mercury (Hg), arsenic (As), and cadmium (Cd). Quantitative real-time PCR was used to assess gene expressions. The findings of this study confirmed that exposure to low concentration heavy metals upregulated N-methyl-D-aspartate (NMDA) receptor subunits NMDAR2A (NR2A), NMDAR2B (NR2B), and NMDAR2D (NR2D) and B cell lymphoma (Bcl-2) genes. NR2A genes were significantly upregulated by 90 and 74%, respectively, on exposure to Pb + As and Pb + Cd. NR2B genes were upregulated by 85.3, 68.6, 62.7, and 62.7% on exposure to As, Pb + Hg, Pb + As, and Pb + Cd, respectively. Exposure to As, Pb + Cd, and Pb + Hg + As significantly upregulated Bcl-2 genes by 2.01-, 1.84-, and 1.80-fold, respectively. NR1A and C-fos gene expressions were not significantly different from control. Upregulation of NMDAR subunits and Bcl-2 genes in this study was largely a counter measure against insults from exposure to low concentration heavy metals. Principal component analysis confirmed the influence of low concentration individual and mixtures of Pb, Hg, As, and Cd on gene expression of NMDAR subunits and Bcl-2. These data suggest that altered expression of NMDA receptor subunits and Bcl-2 genes may explain toxicity of low concentration individual and mixtures of Pb, Hg, As, and Cd.


Similar content being viewed by others
References
Ainza C, Trevors J, Saier M (2010) Environmental mercury rising. Water Air Soil Pollut 205:47–48
Akhtar RS, Ness JM, Roth KA (2004) Bcl-2 family regulation of neuronal development and neurodegeneration. Biochim Biophys Acta 1644:189–203. doi:10.1016/j.bbamcr.2003.10.013
Ali H, Khan E, Sajad MA (2013) Phytoremediation of heavy metals—concepts and applications. Chemosphere 91:869–881
Amsterdam A, Nissen RM, Sun Z, Swindell EC, Farrington S, Hopkins N (2004) Identification of 315 genes essential for early zebrafish development. Proc Natl Acad Sci USA 101:12792–12797
An G, Lin TN, Liu JS, Xue JJ, He YY, Hsu CY (1993) Expression of c-fos and c-jun family genes after focal cerebral ischemia. Ann Neurol 33:457–464
Awofolu O (2005) A survey of trace metals in vegetation, soil and lower animal along some selected major roads in metropolitan city of Lagos. Environ Monitor Assess 105:431–447
Borges V, Santos F, Rocha J, Nogueira C (2007) Heavy metals modulate glutamatergic system in human platelets. Neurochem Res 32:953–958
Cambier S, Gonzalez P, Durrieu G, Bourdineaud J-P (2010) Cadmium-induced genotoxicity in zebrafish at environmentally relevant doses. Ecotoxicol Environ Saf 73:312–319. doi:10.1016/j.ecoenv.2009.10.012
Chan T-M et al (2009) Developmental gene regulatory networks in the zebrafish embryo. Biochim Biophys Acta (BBA)-Gene Regul Mech 1789:279–298
Cherbonnel-Lasserre C, Dosanjh M (1997) Suppression of apoptosis by overexpression of Bcl-2 or Bcl-x L promotes survival and mutagenesis after oxidative damage. Biochimie 79:613–617
Chow ESH, Hui MNY, Lin CC, Cheng SH (2008) Cadmium inhibits neurogenesis in zebrafish embryonic brain development. Aquat Toxicol 87:157–169. doi:10.1016/j.aquatox.2008.01.019
da Silva Acosta D et al (2016) Copper at low levels impairs memory of adult zebrafish (Danio rerio) and affects swimming performance of larvae. Comp Biochem Physiol C Toxicol Pharm 185:122–130
Fan G et al (2010) Methionine choline reverses lead-induced cognitive and N-methyl-D-aspartate receptor subunit 1 deficits. Toxicology 272:23–31. doi:10.1016/j.tox.2010.03.018
Ge H-L, Liu S-S, Su B-X, Qin L-T (2014) Predicting synergistic toxicity of heavy metals and ionic liquids on photobacterium Q67. J Hazard Mat 268:77–83
Gómez-Fernández JC (2014) Functions of the C-terminal domains of apoptosis-related proteins of the Bcl-2 family. Chem Phys Lipids 183:77–90. doi:10.1016/j.chemphyslip.2014.05.003
Guilarte TR, McGlothan JL (1998) Hippocampal NMDA receptor mRNA undergoes subunit specific changes during developmental lead exposure. Brain Res 790:98–107. doi:10.1016/S0006-8993(98)00054-7
Güner U (2016) Behavioral toxicological responses of Zebrafish Danio rerio (F. Hamilton, 1822) after exposed with different concentrations of metal mixtures. Toxicol Lett 258:S297
Iqbal MP (2012) Lead pollution: a risk factor for cardiovascular disease in Asian developing countries. Pak J Pharm Sci 25:289–294
Luo J-H, Qiu Z-Q, Shu W-Q, Y-Y Zhang, Zhang L, J-A Chen (2009) Effects of arsenic exposure from drinking water on spatial memory, ultra-structures and NMDAR gene expression of hippocampus in rats. Toxicol Lett 184:121–125
Luo J-H, Qiu Z-Q, Zhang L, Shu W-Q (2012) Arsenite exposure altered the expression of NMDA receptor and postsynaptic signaling proteins in rat hippocampus. Toxicol Lett 211:39–44
Kalra N, Kumar V (2004) c-Fos is a mediator of the c-myc-induced apoptotic signaling in serum-deprived hepatoma cells via the p38 mitogen-activated protein kinase pathway. J Biol Chem 279:25313–25319
Karri V, Schuhmacher M, Kumar V (2016) Heavy metals (Pb, Cd, As and MeHg) as risk factors for cognitive dysfunction: a general review of metal mixture mechanism in brain. Environ Toxicol Pharm 48:203–213
Kesari VP, Kumar A, Khan PK (2012) Genotoxic potential of arsenic at its reference dose. Ecotoxicol Enviro Saf 80:126–131. doi:10.1016/j.ecoenv.2012.02.018
Lahiri DK, Maloney B, Riyaz Basha M, Wen Ge Y, Zawia NH (2007) How and when environmental agents and dietary factors affect the course of Alzheimer’s disease: the “LEARn” model (latent early-life associated regulation) may explain the triggering of AD. Curr Alzheimer Res 4:219–228
Lau WK, Yeung CW, Lui PW, Cheung LH, Poon NT, Yung KKL (2002) Different trends in modulation of NMDAR1 and NMDAR2B gene expression in cultured cortical and hippocampal neurons after lead exposure. Brain Res 932:10–24. doi:10.1016/S0006-8993(01)03395-9
Li F, Tsien JZ (2009) Memory and the NMDA receptors. N Engl J Med 361:302–303. doi:10.1056/NEJMcibr0902052
Li TY, Zhang X, Wei XP, Liu YF, Qu P, Liu YX, Chen J (2011) Impact of antioxidant vitamins and heavy metal levels at birth on neurodevelopment of children assessed at two years of age. Zhonghua Chin J Pediatr 49:439–444
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408
Marchetti C, Gavazzo P (2005) NMDA receptors as targets of heavy metal interaction and toxicity. Neurotox Res 8:245–258
Marshall K-A, Daniel SE, Cairns N, Jenner P, Halliwell B (1997) Upregulation of the anti-apoptotic protein Bcl-2 may be an early event in neurodegeneration: studies on Parkinson’s and incidental Lewy body disease. Biochem Biophys Res Comm 240:84–87
Matović V, Buha A, Ðukić-Ćosić D, Bulat Z (2015) Insight into the oxidative stress induced by lead and/or cadmium in blood, liver and kidneys. Food Chem Toxicol 78:130–140
Mehta A, Prabhakar M, Kumar P, Deshmukh R, Sharma PL (2013) Excitotoxicity: bridge to various triggers in neurodegenerative disorders. Eur J Pharmacol 698:6–18. doi:10.1016/j.ejphar.2012.10.032
Mejía JJ, Díaz-Barriga F, Calderón J, Ríos C, Jiménez-Capdeville ME (1997) Effects of lead–arsenic combined exposure on central monoaminergic systems. Neurotoxicol Teratol 19:489–497. doi:10.1016/S0892-0362(97)00066-4
Merino D, Bouillet P (2009) The Bcl-2 family in autoimmune and degenerative disorders. Apoptosis 14:570–583. doi:10.1007/s10495-008-0308-4
Milde-Langosch K (2005) The Fos family of transcription factors and their role in tumourigenesis. Eur J Cancer 41:2449–2461. doi:10.1016/j.ejca.2005.08.008
Mony L, Kew JN, Gunthorpe MJ, Paoletti P (2009) Allosteric modulators of NR2B-containing NMDA receptors: molecular mechanisms and therapeutic potential. Br J Pharmacol 157:1301–1317. doi:10.1111/j.1476-5381.2009.00304.x
Nihei MK, Guilarte TR (2001) Molecular changes in glutamatergic synapses induced by Pb2+: association with deficits of LTP and spatial learning. NeuroToxicology 22:635–643. doi:10.1016/S0161-813X(01)00035-3
Pan J-G, Zhang J, Zhou H, Chen L, Tang Y-H, Zheng Y (2011) Protective action of endogenously generated H2S on hypoxia-induced respiratory suppression and its relation to antioxidation and down-regulation of c-fos mRNA in medullary slices of neonatal rats. Resp Physiol Neurobiol 178:230–234. doi:10.1016/j.resp.2011.06.013
Panhwar AH et al (2015) Comparative evaluation of essential and toxic elements in the blood of kidney failure patients and healthy referents. Environ Monit Assess 187:1–11
Paoletti P, Neyton J (2007) NMDA receptor subunits: function and pharmacology. Curr Opin Pharmacol 7:39–47. doi:10.1016/j.coph.2006.08.011
Papadia S, Hardingham GE (2007) The dichotomy of NMDA receptor signaling. Neuroscientist 13:572–579
Powers CM, Yen J, Linney EA, Seidler FJ, Slotkin TA (2010) Silver exposure in developing zebrafish (Danio rerio): persistent effects on larval behavior and survival. Neurotoxicol Teratol 32:391–397. doi:10.1016/j.ntt.2010.01.009
Ramos-Chávez LA, Rendón-López CR, Silva-Adaya D, Zepeda A, Del Razo LM, Gonsebatt ME (2015) Neurological effects of inorganic arsenic exposure: altered cysteine/glutamate transport, NMDA expression and spatial memory impairment. Front Cell Neurosci. doi:10.3389/fncel.2015.00021
Ruszkiewicz J, Albrecht J (2015) Changes in the mitochondrial antioxidant systems in neurodegenerative diseases and acute brain disorders. Neurochem Int 88:66–72
Santillo A, Falvo S, Chieffi P, Burrone L, Chieffi Baccari G, Longobardi S, Di Fiore MM (2014) D-aspartate affects NMDA receptor-extracellular signal–regulated kinase pathway and upregulates androgen receptor expression in the rat testis. Theriogenology 81:744–751. doi:10.1016/j.theriogenology.2013.12.009
Sarkar S, Mukherjee S, Chattopadhyay A, Bhattacharya S (2014) Low dose of arsenic trioxide triggers oxidative stress in zebrafish brain: expression of antioxidant genes. Ecotoxicol Environ Saf 107:1–8
Scimemi A, Tian H, Diamond JS (2009) Neuronal transporters regulate glutamate clearance, NMDA receptor activation and synaptic plasticity in the hippocampus. J Neurosci 29:14581–14595. doi:10.1523/JNEUROSCI.4845-09.2009
Seriani R et al (2015) In vitro mucus transportability, cytogenotoxicity, and hematological changes as non-destructive physiological biomarkers in fish chronically exposed to metals. Ecotoxicol Environ Saf 112:162–168. doi:10.1016/j.ecoenv.2014.11.003
Shacka JJ, Roth KA (2005) Regulation of neuronal cell death and neurodegeneration by members of the Bcl-2 family: therapeutic implications. Curr Drug Targets CNS Neurol Dis 4:25–39
Smith E, Gancarz D, Rofe A, Kempson IM, Weber J, Juhasz AL (2012) Antagonistic effects of cadmium on lead accumulation in pregnant and non-pregnant mice. J Hazard Mat 199:453–456
Sonnack L et al (2015) Effects of metal exposure on motor neuron development, neuromasts and the escape response of zebrafish embryos. Neurotoxicol Teratol 50:33–42
Steinman HM (1995) The Bcl-2 oncoprotein functions as a pro-oxidant. J Biol Chem 270:3487–3490
Stewart AM, Braubach O, Spitsbergen J, Gerlai R, Kalueff AV (2014) Zebrafish models for translational neuroscience research: from tank to bedside. Trends Neurosci 37:264–278
Tang R, Dodd A, Lai D, McNabb WC, Love DR (2007) Validation of Zebrafish (Danio rerio) reference genes for quantitative real-time RT-PCR normalization. Acta Biochim Biophys Sin 39:384–390. doi:10.1111/j.1745-7270.2007.00283.x
Toscano CD, Hashemzadeh-Gargari H, McGlothan JL, Guilarte TR (2002) Developmental Pb2 + exposure alters NMDAR subtypes and reduces CREB phosphorylation in the rat brain. Dev Brain Res 139:217–226. doi:10.1016/S0165-3806(02)00569-2
Tripathi RD, Srivastava S, Mishra S, Singh N, Tuli R, Gupta DK, Maathuis FJ (2007) Arsenic hazards: strategies for tolerance and remediation by plants. Trends Biotechnol 25:158–165
Vidal L, Durán R, Faro LF, Campos F, Cervantes RC, Alfonso M (2007) Protection from inorganic mercury effects on the in vivo dopamine release by ionotropic glutamate receptor antagonists and nitric oxide synthase inhibitors. Toxicology 238:140–146. doi:10.1016/j.tox.2007.05.025
Waxman EA, Lynch DR (2005) N-methyl-D-aspartate receptor subtypes: multiple roles in excitotoxicity and neurological disease. Neuroscientist 11:37–49. doi:10.1177/1073858404269012
Wuana RA, Okieimen FE (2011) Heavy metals in contaminated soils: a review of sources, chemistry, risks and best available strategies for remediation ISRN. Ecology 2011
Xu H, Shao X, Zhang Z, Zou Y, Wu X, Yang L (2013) Oxidative stress and immune related gene expression following exposure to di-n-butyl phthalate and diethyl phthalate in zebrafish embryos. Ecotoxicol Environ Saf 93:39–44. doi:10.1016/j.ecoenv.2013.03.038
Zahran E, Risha E (2014) Modulatory role of dietary Chlorella vulgaris powder against arsenic-induced immunotoxicity and oxidative stress in Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol 41:654–662. doi:10.1016/j.fsi.2014.09.035
Zhu B, Liu L, Li D-L, Ling F, Wang G-X (2014) Developmental toxicity in rare minnow (Gobiocypris rarus) embryos exposed to Cu, Zn and Cd. Ecotoxicol Environ Saf 104:269–277. doi:10.1016/j.ecoenv.2014.03.018
Acknowledgements
This work was supported financially by the Priority Academic Program Development of Jiangsu Higher Education Institutions, Collaborative Innovation Center of Technology and Material of Water Treatment, Specialized Research Fund for the Doctoral Program of Chinese Universities from the Ministry of Education (20113227110020) and Graduate Innovative Projects in Jiangsu Province (KYLX_1067).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cobbina, S.J., Mao, G., Zhao, T. et al. Modulation of N-Methyl-D-Aspartate Receptors (NMDAR), Bcl-2 and C-Fos Gene Expressions on Exposure to Individual and Mixtures of Low Concentration Metals in Zebrafish (Danio rerio). Arch Environ Contam Toxicol 72, 418–427 (2017). https://doi.org/10.1007/s00244-016-0352-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00244-016-0352-y