Abstract
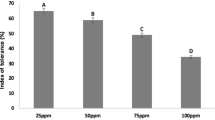
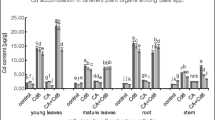
The metabolic response of plants to exogenous supply and bioaccumulation of trivalent chromium (Cr3+ ) was investigated. Pre-rooted young hybrid willows (Salix matsudana Koidz × alba L.) were exposed to hydroponic solution spiked with CrCl3 at 24.0°C ± 1°C for 192 hours. Various physiologic parameters of the plants were monitored to determine toxicity from Cr exposure. The transpiration rate of willows exposed to 2.5 mg Cr/L was 49% higher than that of the untreated control plants, but it was decreased by 17% when exposed to 30.0 mg Cr/L. Significant decrease (≥20%) of soluble protein in young leaves of willows was detected in the treatment group with ≥7.5 mg Cr/L. The measured chlorophyll contents in leaves of treated plants varied with the dose of Cr, but a linear correlation could not be established. The contents of chlorophyll in leaves of willows exposed to ≥7.5 mg Cr/L were higher than that of the untreated plants but lower at 30.0 mg Cr/L. Superoxide dismutase activity (SOD) in leaves between the treated and untreated willows did not show any significant difference, but activities of both catalase (CAT) and peroxidase (POD) in leaf cells of all treated plants were higher than those in the untreated willows. The correlation between the concentration of Cr and CAT activity in leaf cells was the highest of all toxicity assays (R 2 = 0.9096), indicating that CAT activity was most sensitive to the change in Cr3+ doses compared with the other selected parameters. Results from the Cr uptake study showed that significant removal of Cr from hydroponic solution was observed in the presence of hybrid willows without showing detectable phytotoxicity, even at high does of Cr. More than 90% of the applied Cr3+ was removed from the aqueous solution by willows at concentrations up to 7.5 mg Cr/L. Approximately 70% of the initial Cr was recovered in the plant materials. At the low-Cr3+ treatment (2.5 mg Cr/L), Cr accumulation by willow materials was the greatest (92%) in roots and the lowest (0.2%) in leaves, whereas the greatest (66%) was in stems and the lowest (0.1%) in leaves of willows exposed to 30.0 mg Cr/L. The correlation between applied Cr3+ (mg Cr/L) and Cr (μg Cr/g fresh weight [fw]) accumulated in plant materials was significant. The bioaccumulation kinetics of Cr by hybrid willows can be described by a typical saturation curve. Results also indicated that translocation of Cr from roots to shoots was possible. It is to conclude that hybrid willows have great potential as bioremediation technology in the removal of chromium (Cr3+) from contaminated effluents and sediments.



Similar content being viewed by others
References
Anderson RA (1989) Essentiality of chromium in humans. Sci Total Environ 86:75–81
Anderson RA (1997) Chromium as an essential nutrient for humans. Regul Toxicol Pharmacol 26:S35–S41
Banks MK, Schwab AP, Henderson C (2006) Leaching and reduction of chromium in soil as affected by soil organic content and plants. Chemosphere 62:255–264
Becquer T, Quantin C, Sicot M, Boudot JP (2003) Chromium availability in ultramafic soils from New Caledonia. Sci Total Environ 301:251–261
Bonet A, Poschenrieder C, Barcelo J (1991) Chromium III-ion interactions in Fe-deficient and Fe-sufficient bean plants. I. Growth and nutrient content. J Plant Nutr 14:403–414
Boonyapookana B, Upatham S, Kruatrachue M, Pokethitiyook P, Singhakaew S (2002) Phytoaccumulation and phytotoxicity of cadmium and chromium in duckweed Wolffia globosa. Int J Phytoremed 4:87–100
Cheung KH, Gu JD (2005) Chromate reduction by Bacillus megaterium TKW3 isolated from marine sediments. World J Microbiol Biotechnol 21:213–219
Chatterjee J, Chatterjee C (2000) Phytotoxicity of cobalt, chromium and copper in cauliflower. Environ Pollut 109:69–74
Dixit V, Pandey V, Shyam R (2002) Chromium ions inactivate electron transport and enhance superoxide generation in vivo in pea (Pisum sativum L.cv. Azad) root mitochondria. Plant Cell Environ 25:687–693
Fridovich I (1978) The biology of oxygen radical. Science 39:522–526
Fridovich I (1989) Superoxide dismutases: An adaptation to a paramagnetic gas. J Biol Chem 264:7761–7764
Han FX, Banin A, Su Y, Monts DL, Plodinec MJ, Kingery WL, et al. (2002) Industrial age anthropogenic inputs of heavy metals into the pedosphere. Naturwissenschaften 89:497–504
Hauschild MZ (1993) Putrescine (1,4-diaminobutane) as an indicator of pollution-induced stress in higher plants: Barley and rape stressed with Cr(III) or Cr(VI). Ecotoxicol Environ Safe 26:228–247
Hunter JG, Vergnano O (1953) Trace element toxicities in oat plants. Ann Appl Biol 4: 761–777
Jin JH, Ding ZR (1981) Methods of plant biochemistry analysis. Chinese Science Press, Beijing, China
Khan AG (2001) Relationships between chromium biomagnification ratio, accumulation factor, and mycorrhizae in plants growing on tannery effluent-polluted soil. Environ Int 26:417–423
Katz SA, Salem H (1994) The biologic and environmental chemistry of chromium. VCH Publishers, New York, NY
Kimbrough DE, Cohen Y, Winer AM, Creelam L, Mabuni C (1999) A critical assessment of chromium in the environment. Crit Rev Environ Sci Technol 29:1–46
Liu HY, Liao BH, Zhou PH, Yu PZ (2004) Toxicity of linear alkylbenzene sulfonate and alkylethoxylate to aquatic plants. Bull Environ Contam Toxicol 72:866–872
Lytle CM, Lytle FW, Yang N, Qian J, Hansem D, Zayed A, et al. (1998) Reduction of Cr(VI) to Cr(III) by wetland plants: Potential for in situ heavy metal detoxification. Environ Sci Technol 32:3087–3093
Maclachalam S, Zalik S (1963) Plastid structure, chlorophyll concentration and free amino acid composition of a chlorophyll mutant of barley. Can J Bot 41:1053–1062
Marschner H (1995) Mineral nutrition of higher plants, 2nd ed. Academic/Harcourt Brace, New York, NY
Mei BJ, Puryear JD, Newton RJ (2002) Assessment of Cr tolerance and accumulation in selected plant species. Plant Soil 247:223–231
Mishra S, Singh V, Srivastava S, Srivastava R, Srivastava M, Dass S, et al. (1995) Studies on uptake of trivalent and hexavalent Cr by maize (Zea mays). Food Chem Toxic 33:393–397
Panda SK, Choudhury S (2005) Chromium stress in plants. Braz J Plant Physiol 17:95–102
Panda SK, Khan MH (2003) Antioxidant efficiency in rice (Oryza sativa L.) leaves under heavy metal toxicity. J Plant Biol 30:23–29
Pulford ID, Watson C, McGregor SD (2001) Uptake of chromium by trees: Prospects for phytoremediation. Environ Geochem Health 23:307–311
Rai UN, Tripathi RD, Kumar N (1992) Bioaccumulation of chromium and toxicity on growth, photosynthetic pigments, photosynthesis in vivo nitrate reductase activity and protein content in a chlorococcalean green alga Glaucocystis nostochinearum Itzigsohn. Chemosphere 25:721–732
Raskin I, Kumar PBAN, Dushenkov S, Salt D (1994) Bioconcentration of heavy metals by plants. Curr Opin Biotechnol 28:115–126
Reeves RD, Baker AJM, Brooks RR (1996) Abnormal accumulation of trace metals by plants. Mining Environ Manage 3:4–8
Richard FC, Bourg ACM (1991) Aqueous geochemistry of chromium: A review. Water Res 25:807–816
Sachs L (1992) Angewandte statistik. Springer, Berlin, Germany
Scoccianti V, Crinelli R, Tirillini B, Mancinelli V, Speranza A (2006) Uptake and toxicity of Cr (Cr3+) in celery seedlings. Chemosphere 64:1695–1703
Sharma DC, Sharma CP, Tripathi RD (2003) Phytotoxic lesions of chromium in maize. Chemosphere 51:63–68
Shanker AK, Cervantes C, Loza-Tavera H, Avudainayagam S (2005) Chromium toxicity in plants. Environ Int 31:739–753
Skeffington RA, Shewry PR, Perterson PJ (1976) Chromium uptake and transport in barley seedlings (Hordeum vulgare L.). Planta 19:807–810
Tsang E, Bowler C, Herouart D, Villarroel R, Genetello C, Inze D (1991) Differential regulation of superoxide dismutases in plants exposed to environmental stress. Plant Cell 3:783–792
Trapp S, Zambrano KC, Kusk KO, Karlson U (2000) A phytotoxicity test using transpiration of willows. Arch Environ Contam Toxicol 39: 154–160
Vajpayee P, Tripathi RD, Rai UN, Ali MB, Singh SN (2000) Chromium accumulation reduction chlorophyll biosynthesis, nitrate reductase activity and protein content in Nympaea alba L. Chemosphere 41:1075–1082
Xu XR, Li HB, Gu JD (2005) Kinetics of the reduction of chromium (VI) by vitamin C. Environ Toxicol Chem 24:1310–1314
Yu XZ, Trapp S, Zhou PH, Peng XY, Cao X (2006) Response of weeping willows to linear alkylbenzene sulfonate. Chemosphere 64:43–48
Zayed AM, Lytle CM, Qian JH, Terry N (1998) Chromium accumulation, translocation and chemical speciation in vegetable crops. Planta 206:293–299
Zayed AM, Terry N (2003) Chromium in environment: Factors affecting biologic remediation. Plant Soil 249:135–156
Acknowledgments
This work was supported by a doctoral studentship from The University of Hong Kong. Thanks to S.-Z. Huang for technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yu, XZ., Gu, JD. Accumulation and Distribution of Trivalent Chromium and Effects on Hybrid Willow (Salix matsudana Koidz × alba L.) Metabolism. Arch Environ Contam Toxicol 52, 503–511 (2007). https://doi.org/10.1007/s00244-006-0155-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00244-006-0155-7