Abstract
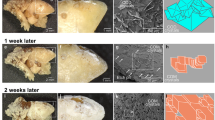
Synthetic calcium phosphate (CaP) concretions in the form of hemispheres formed under carefully controlled conditions (pH 7.4, 37 °C, no evaporation) from stagnant simulated body fluid on anionic polymeric substrate (laminin) were observed by scanning and transmission electron microscopy and atomic force microscopy. The hemispheres with diameter between approximately 50 and 200 μm were composed of closely connected round spherical and elliptical objects of diameter varying from 70 to 120 nm with surface layer composed of tightly packed spherical objects of diameter 25–30 nm. The phase composition of concretions consisting of amorphous material was uniform. The concretions were formed by aggregation of CaP clusters (Posner’s clusters Ca9(PO4)3 or [Ca3(PO4)2] n ) generated in the solution by perikinetic coagulation, their settling onto a substrate and subsequent accumulation through surface migration (surface nucleation) followed by accretion of nanoparticles arriving from surrounding solution. The similarity of ultrafine structures of these synthetic CaP concretions and the compact phosphatic phase appearing in some phosphate calculi indicates that analogous mechanisms could be active at the formation of the latter in the kidneys.




Similar content being viewed by others
References
Grases F, Costa Bauza A, Prieto RM, Gomila I, Pieras E, Söhnel O (2011) Non-infectious phosphate renal calculi: fine structure, chemical and phase composition. Scand J Clin Lab Investig 71:407–412
Carpentier X, Daudon M, Traxer O, Jungers P, Mazouyes A, Matzem G, Veron E, Bazin D (2009) Relationships between carbonation rate of carbapatite and morphologic Characteristics of calcium phosphate stones and etiology. Urology 73(5):968–975
Meyer AS, Finlayson B, DuBois L (1971) Direct observation of urinary stone Ultrastructure. Brit J Urol 43:154–163
Grases F, Söhnel O, Vilacampa AI, March JG (1996) Phosphates precipitating from artificial urine and fine structure of phosphate renal calculi. Clin Chim Acta 244:45–67
Kim KM (1982) The stones. Scanning Electron Microscopy (Pt4):1635–1660
Zelenková M, Söhnel O, Grases F (2012) Ultra-fine structure of the hydroxyapatite amorphous phase in non-infectious phosphate renal calculi. Urology
Söhnel O, Grases F (2011) Supersaturation of body fluids, plasma and urine, with respect to biological hydroxyapatite. Urol Res 39:429–436
Li J, Liao H, Sjostrom M (1997) Characterization of calcium phosphates precipitated from simulated body fluid of different buffering capacities. Biomaterials 18:743–747
Lu X, Yang L (2005) Theoretical analysis of calcium phosphate precipitation in simulated body fluid. Biomaterials 26:1097–1108
Kanzaki N, Treboux G, Onma K, Tsutsumi S, Ito A (2001) Calcium phosphate clusters. Biomaterials 22:2921–2929
Ganesan K, Apple M (2008) Calcium phosphate nanoparticles as nuclei for the preparation of collodial calcium phytate. New J Chem 23:1326–1330
Onuma K, Ito A (1998) Cluster growth model for hydroxyapatite. Chem Mater 10:3346–3351
Oyane A, Onuma K, Kokubo T, Ito A (1999) Clustering of calcium phosphate in the system CaCl2-H3PO4-KCl-H2O. J Phys Chem B 15:6557–6562
Söhnel O, Garside J (1992) Precipitation: Basic principles and Industrial Applications. Butterworth, Oxford, p 145, p 187. ISBN 0 7056 1107 3
Suemitsu M, Togashi H, Abe T (2003) Autocatalytic reaction model: a phenomenology for nucleation coalescence-growth of thin films. Thin Solid Films 428:83–86
Petrov I, Barna PB, Hulman L, Greene JE (2003) Microstructural evolution during film growth. J Vac Sci Technik 21:S117–S128
Rhee S-H, Tahala J (1998) Hydroxyapatite coating on a colagen membrane by a biomimetic method. J Am Ceram Soc 81:3029–3031
Rhee S-H, Lee JD, Tanaka J (2000) Nucleation of hydroxyapatite through chemical interaction with colagen. J Am Ceram Soc 83:2890–2892
Takeuchi A, Ohtsuki Ch, Miyazaki T et al (2005) Heterogeneous nucleation of hydroxyapatite on protein:structural effect of silk sericin. J R Soc Interface 2:373–378
Feenstra TP, de Bruyn PL (1980) Light scattering studies on solutions containing calcuim phosphate. J Coll Interface Sci 71:431–437
Kibalczyc W, Bondarczuk K (1985) Light scattering study of calcium phosphate Precipitation. J Cyst Growth 71:751–756
Koutsopoulos S (2001) Kinetic study on the crystal growth of hydroxyapatite. Langmuir 17:8092–8097
Ganesan K, Epple M (2008) Calcium phosphate nanoparticles as nuclei for the preparation of colloidal calcium phytate. New J Chem 32:1326–1330
Matsui I, Hamano T, Mikami S, Fuji N, Takabatake Y, Nagasawa Y, Kawada N, Ito T, Rakugi H, Imai E, Isaka Y (2009) Fully phosphorilated fetuin-A forms a mineral complex in the serum of rats with adenine-induced renal failure. Kidney Int 75(9):915–928
Pasch A, Farese S, Graber S, Wald J, Richtering W, Floege J, Jahnen-Dechent W (2012) Nanoparticle-based test measures overall propensity for calcification in serum. J Am Soc Nephrol 23(10):1744–1752
Acknowledgments
This work was supported by the project CTQ2010-18271/PPQ from t Ministerio de Ciencia e Innovación (Gobierno de España), FEDER funds (European Union) and the project grant 9/2011 from the Conselleria d’Educació, Cultura i Universitat (Govern de les Illes Balears).
Conflict of interest
The authors declare that they have no competing interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Grases, F., Zelenková, M. & Söhnel, O. Structure and formation mechanism of calcium phosphate concretions formed in simulated body fluid. Urolithiasis 42, 9–16 (2014). https://doi.org/10.1007/s00240-013-0611-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00240-013-0611-6