Abstract
Purpose
Nearly all literature for predicting tumor grade in astrocytoma and oligodendroglioma pre-dates the molecular classification system. We investigated the association between contrast enhancement, ADC, and rCBV with tumor grade separately for IDH-mutant astrocytomas and molecularly-defined oligodendrogliomas.
Methods
For this retrospective study, 44 patients with IDH-mutant astrocytomas (WHO grades II, III, or IV) and 39 patients with oligodendrogliomas (IDH-mutant and 1p/19q codeleted) (WHO grade II or III) were enrolled. Two readers independently assessed preoperative MRI for contrast enhancement, ADC, and rCBV. Inter-reader agreement was calculated, and statistical associations between MRI metrics and WHO grade were determined per reader.
Results
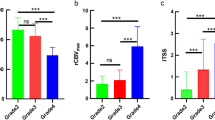
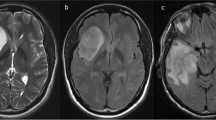
For IDH-mutant astrocytomas, both readers found a stepwise positive association between contrast enhancement and WHO grade (Reader A: OR 7.79 [1.97, 30.80], p = 0.003; Reader B: OR 6.62 [1.70, 25.82], p = 0.006); both readers found that ADC was negatively associated with WHO grade (Reader A: OR 0.74 [0.61, 0.90], p = 0.002); Reader B: OR 0.80 [0.66, 0.96], p = 0.017), and both readers found that rCBV was positively associated with WHO grade (Reader A: OR 2.33 [1.35, 4.00], p = 0.002; Reader B: OR 2.13 [1.30, 3.57], p = 0.003). For oligodendrogliomas, both readers found a positive association between contrast enhancement and WHO grade (Reader A: OR 15.33 [2.56, 91.95], p = 0.003; Reader B: OR 20.00 [2.19, 182.45], p = 0.008), but neither reader found an association between ADC or rCBV and WHO grade.
Conclusions
Contrast enhancement predicts WHO grade for IDH-mutant astrocytomas and oligodendrogliomas. ADC and rCBV predict WHO grade for IDH-mutant astrocytomas, but not for oligodendrogliomas.


Similar content being viewed by others
References
Louis DN, Perry A, Reifenberger G et al (2016) The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol 131:803–820. https://doi.org/10.1007/s00401-016-1545-1
The Cancer Genome Atlas Research Network (2015) Comprehensive, integrative genomic analysis of diffuse lower-grade gliomas. N Engl J Med 372:2481–2498. https://doi.org/10.1056/NEJMoa1402121
Ceccarelli M, Barthel FP, Malta TM et al (2016) Molecular profiling reveals biologically discrete subsets and pathways of progression in diffuse glioma. Cell 164:550–563. https://doi.org/10.1016/j.cell.2015.12.028
Eckel-Passow JE, Lachance DH, Molinaro AM et al (2015) Glioma groups based on 1p/19q, IDH, and TERT promoter mutations in tumors. N Engl J Med 372:2499–2508. https://doi.org/10.1056/NEJMoa1407279
Patel SH, Poisson LM, Brat DJ et al (2017) T2-FLAIR mismatch, an imaging biomarker for IDH and 1p/19q status in lower-grade gliomas: a TCGA/TCIA project. Clin Cancer Res 23:6078–6085. https://doi.org/10.1158/1078-0432.CCR-17-0560
Jain R, Johnson DR, Patel SH et al (2020) “Real world” use of a highly reliable imaging sign: “T2-FLAIR mismatch” for identification of IDH mutant astrocytomas. Neuro Oncol 22:936–943. https://doi.org/10.1093/neuonc/noaa041
Aliotta E, Dutta SW, Feng X et al (2020) Automated apparent diffusion coefficient analysis for genotype prediction in lower grade glioma: association with the T2-FLAIR mismatch sign. J Neurooncol 149:325–335. https://doi.org/10.1007/s11060-020-03611-8
Reuss DE, Mamatjan Y, Schrimpf D et al (2015) IDH mutant diffuse and anaplastic astrocytomas have similar age at presentation and little difference in survival: a grading problem for WHO. Acta Neuropathol 129:867–873. https://doi.org/10.1007/s00401-015-1438-8
Hartmann C, Hentschel B, Wick W et al (2010) Patients with IDH1 wild type anaplastic astrocytomas exhibit worse prognosis than IDH1-mutated glioblastomas, and IDH1 mutation status accounts for the unfavorable prognostic effect of higher age: implications for classification of gliomas. Acta Neuropathol 120:707–718. https://doi.org/10.1007/s00401-010-0781-z
Shaw E, Arusell R, Scheithauer B et al (2002) Prospective randomized trial of low- versus high-dose radiation therapy in adults with supratentorial low-grade glioma: initial report of a North Central Cancer Treatment Group/Radiation Therapy Oncology Group/Eastern Cooperative Oncology Group study. J Clin Oncol 20:2267–2276. https://doi.org/10.1200/JCO.2002.09.126
Stupp R, Mason WP, van den Bent MJ et al (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352:987–996. https://doi.org/10.1056/NEJMoa043330
Yeboa DN, Yu JB, Liao E et al (2019) Differences in patterns of care and outcomes between grade II and grade III molecularly defined 1p19q co-deleted gliomas. Clin Transl Radiat Oncol 15:46–52. https://doi.org/10.1016/j.ctro.2018.12.003
Franceschi E, Tosoni A, Bartolini S et al (2020) Histopathological grading affects survival in patients with IDH-mutant grade II and grade III diffuse gliomas. Eur J Cancer 137:10–17. https://doi.org/10.1016/j.ejca.2020.06.018
Mair MJ, Geurts M, van den Bent MJ, Berghoff AS (2021) A basic review on systemic treatment options in WHO grade II-III gliomas. Cancer Treat Rev 92:102124. https://doi.org/10.1016/j.ctrv.2020.102124
Buckner JC, Shaw EG, Pugh SL et al (2016) Radiation plus procarbazine, CCNU, and vincristine in low-grade glioma. N Engl J Med 374:1344–1355. https://doi.org/10.1056/NEJMoa1500925
van den Bent MJ, Brandes AA, Taphoorn MJB et al (2013) Adjuvant procarbazine, lomustine, and vincristine chemotherapy in newly diagnosed anaplastic oligodendroglioma: long-term follow-up of EORTC brain tumor group study 26951. J Clin Oncol 31:344–350. https://doi.org/10.1200/JCO.2012.43.2229
van den Bent MJ (2010) Interobserver variation of the histopathological diagnosis in clinical trials on glioma: a clinician’s perspective. Acta Neuropathol 120:297–304. https://doi.org/10.1007/s00401-010-0725-7
Dean BL, Drayer BP, Bird CR et al (1990) Gliomas: classification with MR imaging. Radiology 174:411–415. https://doi.org/10.1148/radiology.174.2.2153310
Watanabe M, Tanaka R, Takeda N (1992) Magnetic resonance imaging and histopathology of cerebral gliomas. Neuroradiology 34:463–469. https://doi.org/10.1007/BF00598951
Wetzel SG, Cha S, Johnson G et al (2002) Relative cerebral blood volume measurements in intracranial mass lesions: interobserver and intraobserver reproducibility study. Radiology 224:797–803. https://doi.org/10.1148/radiol.2243011014
Shin JH, Lee HK, Kwun BD et al (2002) Using relative cerebral blood flow and volume to evaluate the histopathologic grade of cerebral gliomas: preliminary results. Am J Roentgenol 179:783–789. https://doi.org/10.2214/ajr.179.3.1790783
Law M, Yang S, Wang H et al (2003) Glioma grading: sensitivity, specificity, and predictive values of perfusion MR imaging and proton MR spectroscopic imaging compared with conventional MR imaging. AJNR: Am J Neuroradiol 24:1989
Knopp EA, Cha S, Johnson G et al (1999) Glial neoplasms: dynamic contrast-enhanced T2*-weighted MR imaging. Radiology 211:791–798. https://doi.org/10.1148/radiology.211.3.r99jn46791
Law M, Yang S, Babb JS et al (2004) Comparison of cerebral blood volume and vascular permeability from dynamic susceptibility contrast-enhanced perfusion MR imaging with glioma grade. AJNR Am J Neuroradiol 25:746–755
Aronen HJ, Gazit IE, Louis DN et al (1994) Cerebral blood volume maps of gliomas: comparison with tumor grade and histologic findings. Radiology 191:41–51. https://doi.org/10.1148/radiology.191.1.8134596
Higano S, Yun X, Kumabe T et al (2006) Malignant astrocytic tumors: clinical importance of apparent diffusion coefficient in prediction of grade and prognosis. Radiology 241:839–846. https://doi.org/10.1148/radiol.2413051276
Capper D, Weissert S, Balss J et al (2010) Characterization of R132H mutation-specific IDH1 antibody binding in brain tumors. Brain Pathol 20:245–254. https://doi.org/10.1111/j.1750-3639.2009.00352.x
Capper D, Zentgraf H, Balss J et al (2009) Monoclonal antibody specific for IDH1 R132H mutation. Acta Neuropathol 118:599. https://doi.org/10.1007/s00401-009-0595-z
Felsberg J, Wolter M, Seul H et al (2010) Rapid and sensitive assessment of the IDH1 and IDH2 mutation status in cerebral gliomas based on DNA pyrosequencing. Acta Neuropathol 119:501–507. https://doi.org/10.1007/s00401-010-0647-4
Aliotta E, Nourzadeh H, Batchala PP et al (2019) Molecular subtype classification in lower-grade glioma with accelerated DTI. Am J Neuroradiol 40:1458–1463. https://doi.org/10.3174/ajnr.A6162
Patel SH, Batchala PP, Muttikkal TJE et al (2021) Fluid attenuation in non-contrast-enhancing tumor (nCET): an MRI Marker for Isocitrate dehydrogenase (IDH) mutation in glioblastoma. J Neurooncol 152:523–531. https://doi.org/10.1007/s11060-021-03720-y
Carrillo JA, Lai A, Nghiemphu PL et al (2012) Relationship between tumor enhancement, edema, IDH1 mutational status, MGMT promoter methylation, and survival in glioblastoma. AJNR Am J Neuroradiol 33:1349–1355. https://doi.org/10.3174/ajnr.A2950
Suh CH, Kim HS, Jung SC et al (2018) 2-Hydroxyglutarate MR spectroscopy for prediction of isocitrate dehydrogenase mutant glioma: a systemic review and meta-analysis using individual patient data. Neuro Oncol 20:1573–1583. https://doi.org/10.1093/neuonc/noy113
Johnson DR, Diehn FE, Giannini C et al (2017) Genetically defined oligodendroglioma is characterized by indistinct tumor borders at MRI. AJNR Am J Neuroradiol 38:678–684. https://doi.org/10.3174/ajnr.A5070
Saito T, Muragaki Y, Maruyama T et al (2016) Calcification on CT is a simple and valuable preoperative indicator of 1p/19q loss of heterozygosity in supratentorial brain tumors that are suspected grade II and III gliomas. Brain Tumor Pathol 33:175–182. https://doi.org/10.1007/s10014-016-0249-5
Darvishi P, Batchala PP, Patrie JT et al (2020) Prognostic value of preoperative MRI metrics for diffuse lower-grade glioma molecular subtypes. Am J Neuroradiol 41:815–821. https://doi.org/10.3174/ajnr.A6511
Suchorska B, Schüller U, Biczok A et al (2019) Contrast enhancement is a prognostic factor in IDH1/2 mutant, but not in wild-type WHO grade II/III glioma as confirmed by machine learning. Eur J Cancer 107:15–27. https://doi.org/10.1016/j.ejca.2018.10.019
Lin Y, Xing Z, She D et al (2017) IDH mutant and 1p/19q co-deleted oligodendrogliomas: tumor grade stratification using diffusion-, susceptibility-, and perfusion-weighted MRI. Neuroradiology 59:555–562. https://doi.org/10.1007/s00234-017-1839-6
Wu C-C, Jain R, Radmanesh A et al (2018) Predicting genotype and survival in glioma using standard clinical MR imaging apparent diffusion coefficient images: a pilot study from the Cancer Genome Atlas. Am J Neuroradiol 39:1814–1820. https://doi.org/10.3174/ajnr.A5794
Delgado AF, Delgado AF (2017) Discrimination between glioma grades II and III using dynamic susceptibility perfusion MRI: a meta-analysis. Am J Neuroradiol 38:1348–1355. https://doi.org/10.3174/ajnr.A5218
Wu C-C, Jain R, Neto L et al (2019) MR imaging phenotype correlates with extent of genome-wide copy number abundance in IDH mutant gliomas. Neuroradiology 61:1023–1031. https://doi.org/10.1007/s00234-019-02219-8
Yang X, Xing Z, She D et al (2022) Grading of IDH-mutant astrocytoma using diffusion, susceptibility and perfusion-weighted imaging. BMC Med Imaging 22(1):105
Khalid L, Carone M, Dumrongpisutikul N et al (2012) Imaging Characteristics of oligodendrogliomas that predict grade. Am J Neuroradiol 33:852–857. https://doi.org/10.3174/ajnr.A2895
Lev MH, Ozsunar Y, Henson JW et al (2004) Glial tumor grading and outcome prediction using dynamic spin-echo MR susceptibility mapping compared with conventional contrast-enhanced MR: confounding effect of elevated rCBV of oligodendroglimoas. Am J Neuroradiol 25:214–221
Spampinato MV, Smith JK, Kwock L et al (2007) Cerebral blood volume measurements and proton MR spectroscopy in grading of oligodendroglial tumors. AJR Am J Roentgenol 188:204–212. https://doi.org/10.2214/AJR.05.1177
Xu M, See SJ, Ng WH et al (2005) Comparison of magnetic resonance spectroscopy and perfusion-weighted imaging in presurgical grading of oligodendroglial tumors. Neurosurgery 56:919–926 (discussion 919-926)
Aboud O, Shah R, Vera E et al (2022) Challenges of imaging interpretation to predict oligodendroglioma grade: a report from the Neuro-Oncology Branch. CNS Oncol. 11(1):CNS83. https://doi.org/10.2217/cns-2021-0005
Louis DN, Perry A, Wesseling P et al (2021) The 2021 WHO Classification of Tumors of the Central Nervous System: a summary. Neuro Oncol 23:1231–1251. https://doi.org/10.1093/neuonc/noab106
Funding
No funds, grants, or other support was received
The authors have no relevant financial or non-financial interests to disclose.
Author information
Authors and Affiliations
Contributions
David Joyner: study design, implementation, data collection, interpretation, manuscript draft, and revisions
John Garrett: data collection, manuscript draft, and revisions
Prem Batchala: data interpretation, manuscript draft, and revisions
Bharath Rama: manuscript draft and revisions
Joshua Ravicz: manuscript draft and revisions
James Patrie: statistical analysis, interpretation, manuscript draft, and revisions
Maria-B. Lopes: interpretation, manuscript draft, and revisions
Camilo Fadul: interpretation, manuscript draft, and revisions
David Schiff: interpretation, manuscript draft, and revisions
Rajan Jain: interpretation, manuscript draft, and revisions
Sohil Patel: study design, implementation, data collection, interpretation, manuscript draft, and revisions
All the authors have read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Declarations
This research study was conducted retrospectively from data obtained for clinical purposes, in accordance with the guidelines of the institutional IRB. The requirement for informed consent was waived by our IRB.
Conflict of interest
We declare that we have no conflict of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Joyner, D.A., Garrett, J., Batchala, P.P. et al. MRI features predict tumor grade in isocitrate dehydrogenase (IDH)–mutant astrocytoma and oligodendroglioma. Neuroradiology 65, 121–129 (2023). https://doi.org/10.1007/s00234-022-03038-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-022-03038-0