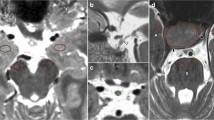
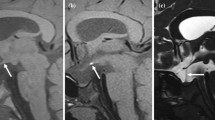
Abstract
Purpose
T2 hypointense signal at the posterior edge of the adenohypophysis (T2HSPA) on magnetic resonance imaging (MRI) is incidentally encountered. We aimed to investigate the prevalence and morphology of T2HSPA and their relationship to age.
Methods
A total of 212 cases between 3 and 88 years old were examined. Sagittal T2-weighted image (T2WI) was evaluated for the presence of T2HSPA, which classified by its morphology into two types (belt-like or nodal). The Wilcoxon rank sum test and chi-square test were used to evaluate the differences between the groups. The T2HSPA was extracted by ImageJ software and measured as a cross-sectional area (CSA) quantitatively by threshold setting. We examined the relationship between CSA of T2HSPA and age, and Spearman’s correlation coefficients were used for statistical analysis.
Results
Of the 212 cases, 80 (37.7%) were identified with T2HSPA. The groups with T2HSPA were significantly younger than the groups without it (p = .01). Groups with belt-like T2HSPA were significantly younger than the groups with nodal T2HSPA (p = .01). There was a weak negative correlation between CSA of T2HSPA and age (p = .02).
Conclusion
T2HSPAs were incidentally detected in 37.7% of all cases, tended to be more common in younger cases, and their morphology was related to age. They seem to have little clinical significance as they tend to decrease in size with age.





Similar content being viewed by others
References
Escourolle H, Abecassis JP, Bertagna X, Guilhaume B, Pariente D, Derome P, Bonnin A, Luton JP (1993) Comparison of computerized tomography and magnetic resonance imaging for the examination of the pituitary gland in patients with Cushing’s disease. Clin Endocrinol (Oxf) 39:307–313. https://doi.org/10.1111/j.1365-2265.1993.tb02370.x
Dwyer AJ, Frank JA, Doppman JL, Oldfield EH, Hickey AM, Cutler GB, Loriaux DL, Schiable TF (1987) Pituitary adenomas in patients with Cushing disease: initial experience with Gd-DTPA-enhanced MR imaging. Radiology 163:421–426. https://doi.org/10.1148/radiology.163.2.3562821
Wolfsberger S, Ba-Ssalamah A, Pinker K, Mlynárik V, Czech T, Knosp E, Trattnig S (2004) Application of three-tesla magnetic resonance imaging for diagnosis and surgery of sellar lesions. J Neurosurg 100:278–286. https://doi.org/10.3171/jns.2004.100.2.0278
Sasaki M, Inoue T, Tohyama K, Oikawa H, Ehara S, Ogawa A (2003) High-field MRI of the central nervous system: current approaches to clinical and microscopic imaging. Magn Reson Med Sci 2:133–139. https://doi.org/10.2463/mrms.2.133
Wolpert SM, Osborne M, Anderson M, Runge VM (1988) The bright pituitary gland—a normal MR appearance in infancy. AJNR Am J Neuroradiol 9:1–3
Cox TD, Elster AD (1991) Normal pituitary gland: changes in shape, size, and signal intensity during the 1st year of life at MR imaging. Radiology 179:721–724. https://doi.org/10.1148/radiology.179.3.2027981
Dietrich RB, Lis LE, Greensite FS, Pitt D (1995) Normal MR appearance of the pituitary gland in the first 2 years of life. AJNR Am J Neuroradiol 16:1413–1419
Miki Y, Asato R, Okumura R, Togashi K, Kimura I, Kawakami S, Konishi J (1993) Anterior pituitary gland in pregnancy: hyperintensity at MR. Radiology 187:229–231. https://doi.org/10.1148/radiology.187.1.8451418
Johnsen DE, Woodruff WW, Allen IS, Cera PJ, Funkhouser GR, Coleman LL (1991) MR imaging of the sellar and juxtasellar regions. Radiographics 11:727–758. https://doi.org/10.1148/radiographics.11.5.1947311
Brassier G, Morandi X, Tayiar E, Riffaud L, Chabert E, Heresbach N, Poirier JY, Carsin-Nicol B (1999) Rathke’s cleft cysts: surgical-MRI correlation in 16 symptomatic cases. J Neuroradiol 26:162–171
Hayashi Y, Tachibana O, Muramatsu N, Tsuchiya H, Tada M, Arakawa Y, Suzuki M, Yamashita J (1999) Rathke cleft cyst: MR and biomedical analysis of cyst content. J Comput Assist Tomogr 23:34–38. https://doi.org/10.1097/00004728-199901000-00008
Ozoner B, Aydin S, Akgun MY, Durmaz ES, Sahin S, Gazioglu N, Kizilkilic O, Kadioglu P, Tanriover N (2019) Predictive factors for Rathke’s cleft cyst consistency. World Neurosurg 128:e522–e530. https://doi.org/10.1016/j.wneu.2019.04.188
Wen L, Hu LB, Feng XY, Desai G, Zou LG, Wang WX, Zhang D (2010) Rathke’s cleft cyst: clinicopathological and MRI findings in 22 patients. Clin Radiol 65:47–55. https://doi.org/10.1016/j.crad.2009.09.010
Güneş A, ÖzbalGüneş S (2020) The neuroimaging features of Rathke’s cleft cysts in children with endocrine-related diseases. Diagn Interv Radiol 26:61–67. https://doi.org/10.5152/dir.2019.19352
Schmidt B, Cattin F, Aubry S (2020) Prevalence of Rathke cleft cysts in children on magnetic resonance imaging. Diagn Interv Imaging 101:209–215. https://doi.org/10.1016/j.diii.2019.12.005
Mahdi ES, Webb RL, Whitehead MT (2019) Prevalence of pituitary cysts in children using modern magnetic resonance imaging techniques. Pediatr Radiol 49:1781–1787. https://doi.org/10.1007/s00247-019-04479-1
Spampinato MV, Castillo M (2005) Congenital pathology of the pituitary gland and parasellar region. Top Magn Reson Imaging 16:269–276. https://doi.org/10.1097/01.rmr.0000224683.98876.51
Schroeder JW, Vezina LG (2011) Pediatric sellar and suprasellar lesions. Pediatr Radiol 41:287–298; quiz 404. https://doi.org/10.1007/s00247-010-1968-0
Asa SL, Kovacs K (1984) Functional morphology of the human fetal pituitary. Pathol Annu 19:275–315
Asa SL, Perry A (2020) Tumors of the pituitary gland. AFIP Atlas of tumor and nontumor pathology, series 5. Fascicle 1. Arlington, VA
Voelker JL, Campbell RL, Muller J (1991) Clinical, radiographic, and pathological features of symptomatic Rathke’s cleft cysts. J Neurosurg 74:535–544. https://doi.org/10.3171/jns.1991.74.4.0535
Reneman L, de Win MM, Booij J, van den Brink W, den Heeten GJ, Freling N, Majoie CB (2012) Incidental head and neck findings on MRI in young healthy volunteers: prevalence and clinical implications. AJNR Am J Neuroradiol 33:1971–1974. https://doi.org/10.3174/ajnr.A3217
Kucharczyk W, Peck WW, Kelly WM, Norman D, Newton TH (1987) Rathke cleft cysts: CT, MR imaging, and pathologic features. Radiology 165:491–495. https://doi.org/10.1148/radiology.165.2.3659372
Nemoto Y, Inoue Y, Fukuda T, Shakudo M, Katsuyama J, Hakuba A, Nishimura S, Onoyama Y (1988) MR appearance of Rathke’s cleft cysts. Neuroradiology 30:155–159. https://doi.org/10.1007/BF00395617
Larkin S, Ansorge O (2017) Development and microscopic anatomy of the pituitary gland mdtext.com. https://www.ncbi.nlm.nih.gov/books/NBK425703/. Accessed 13 Nov 2021
Fager CA, Carter H (1966) Intrasellar epithelial cysts. J Neurosurg 24:77–81. https://doi.org/10.3171/jns.1966.24.1.0077
McGrath P (1971) Cysts of sellar and pharyngeal hypophyses. Pathology 3:123–131. https://doi.org/10.3109/00313027109071331
Shanklin WM (1949) On the presence of cysts in the human pituitary. Anat Rec 104:379–407. https://doi.org/10.1002/ar.1091040402
Teramoto A, Hirakawa K, Sanno N, Osamura Y (1994) Incidental pituitary lesions in 1,000 unselected autopsy specimens. Radiology 193:161–164. https://doi.org/10.1148/radiology.193.1.8090885
Harrison MJ, Morgello S, Post KD (1994) Epithelial cystic lesions of the sellar and parasellar region: a continuum of ectodermal derivatives? J Neurosurg 80:1018–1025
Guduk M, Sun HI, Sav MA, Berkman Z (2017) Pituitary colloid cyst. J Craniofac Surg 28:e166–e168. https://doi.org/10.1097/SCS.0000000000003142
Liu Z, Zhang Y, Feng R, Tian Z, Rao Y, Lu Y, Xu J (2019) Intrasellar symptomatic salivary gland: case series and literature review. Pituitary 22:640–646. https://doi.org/10.1007/s11102-019-01002-5
Rittierodt M, Hori A (2007) Pre-morbid morphological conditions of the human pituitary. Neuropathology 27:43–48. https://doi.org/10.1111/j.1440-1789.2006.00745.x
Funding
This research did not receive any specific grant from founding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
Aiko Gobara, Takashi Katsube, Hiroya Asou, Rika Yoshida, Takeshi Yoshizako, and Hajime Kitagaki contributed to the study conception and design. Data collection and analysis were performed by Aiko Gobara, Takashi Katsube, Hiroya Asou, and Rika Yoshida. The first draft of the manuscript was written by Aiko Gobara and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in reports involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gobara, A., Katsube, T., Asou, H. et al. T2 hypointense signal discovered incidentally at the posterior edge of the adenohypophysis on MRI: its prevalence and morphology and their relationship to age. Neuroradiology 64, 1755–1761 (2022). https://doi.org/10.1007/s00234-022-02935-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-022-02935-8