Abstract
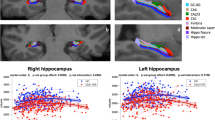
Hippocampal shape anomaly (HSA), characterised by a rounded hippocampus, has been documented in congenital malformations and epileptic patients. Subtle structural hippocampal abnormalities have been demonstrated in patients with schizophrenia. We tested the hypothesis that HSA is more frequent in schizophrenia, particularly in patients from families multiply affected by schizophrenia, and that HSA is transmitted within these families. We also aimed to define the anatomical features of the hippocampus and other cerebral structures in the HSA spectrum and to determine the prevalence of HSA in a control group. We reviewed the magnetic resonance imaging of a large number of subjects with schizophrenia and bipolar disorder, many of who came from multiply affected families, relatives of the affected probands, and controls. Quantitative measures of hippocampal shape and position and other qualitative anatomical measures were performed (including depth of dominant sulcus cortical cap, angle of dominant sulcus and hippocampal fissure, bulk of collateral white matter, prominence of temporal horn lateral recess and blurring of internal hippocampal architecture) on subjects with HSA. A spectrum of mild, moderate and severe HSA was defined. The prevalence of HSA was, 7.8% for the controls (n=218), 9.3% for all schizophrenic subjects (n=151) and 12.3% for familial schizophrenic subjects (n=57). There was a greater prevalence of moderate or severe forms of HSA in familial schizophrenics than controls. However, there was no increase in the prevalence of HSA in the unaffected first-degree relatives of schizophrenic patients or in patients with familial bipolar disorder. HSA was rarely transmitted in families. HSA was frequently associated with a deep, vertical collateral/occipito-temporal sulcus and a steep hippocampal fissure. Our data raise the possibility that HSA is linked to disturbances of certain neurodevelopmental genes associated with schizophrenia. However, the lack of any increase in prevalence in the unaffected relatives of patients and the lack of clustering within individual pedigrees argues against this developmental anomaly being commonly associated with genetic predisposition to the illness.





Similar content being viewed by others
References
Atlas SW, Zimmerman RA, Bilaniuk LT, Rorke L, Hackney DB, Goldberg HI, Grossman RI (1986) Corpus callosum and limbic system: neuroanatomic MR evaluation of developmental anomalies. Radiology 160:355–362
Baker LL, Barkovich AJ (1992) The large temporal horn: MR analysis in developmental brain anomalies versus hydrocephalus. AJNR Am J Neuroradiol 13:115–122
Sato N, Hatakeyama S, Shimizu N, Hikima A, Aoki J, Endo K (2001) MR evaluation of the hippocampus in patients with congenital malformations of the brain. AJNR Am J Neuroradiol 22:389–393
Barsi P, Kenez J, Solymosi D et al (2000) Hippocampal malrotation with normal corpus callosum: a new entity? Neuroradiology 42:339–345
Baulac M, De Grissac N, Hasboun D et al (1998) Hippocampal developmental changes in patients with partial epilepsy: magnetic resonance imaging and clinical aspects. Ann Neurol 44:223–233
Lehericy S, Dormont D, Clemenceau S, Granat O, Marsault C, Baulac M (1995) Developmental abnormalities of the medial temporal lobe in patients with temporal lobe epilepsy. AJNR Am J Neuroradiol 16:617–626
Bogerts B (1997) The temporolimbic system theory of positive schizophrenic symptoms. Schizophr Bull 23:423–435
Csernansky JG, Joshi S, Wang L et al (1998) Hippocampal morphometry in schizophrenia by high dimensional brain mapping. Proc Natl Acad Sci USA 95:11406–11411
Wang L, Joshi SC, Miller MI, Csernansky JG (2001) Statistical analysis of hippocampal asymmetry in schizophrenia. Neuroimage 14:531–545
Shenton ME, Gerig G, McCarley RW, Szekely G, Kikinis R (2002) Amygdala-hippocampal shape differences in schizophrenia: the application of 3D shape models to volumetric MR data. Psychiatry Res 115:15–35
Vogeley K, Tepest R, Pfeifer U et al (2001) Right frontal hypergyria differentiation on affected and unaffected siblings with families multiply affected with schizophrenia: a morphometric MRI study. Am J Psychiatry 158:494–496
Van Os J, Woodruff PWR, Fananas L et al (2000) Association between cerebral structural abnormalities and dermatoglyphic ridge counts in schizophrenia. Comp Psychiatry 41:380–384
Honer WG, Bassett AS, Smith GN, Lapointe JS, Falkai P (1994) Temporal lobe abnormalities in multigenerational families with schizophrenia. Biol Psychiatry 36:737–743
Frangou S, Sharma T, Barta P, Pearlson G, Murray RM (1997) The Maudsley Family Study 4. Normal planum temporale asymmetry in familial schizophrenia. Br J Psychiatry 170:328–333
Sharma T, Lancaster E, Lee D et al (1998) Brain changes in schizophrenia: volumetric MRI study of families multiply affected with schizophrenia—the Maudsley Family Study 5. Br J Psychiatry 173:132–138
McDonald C, Grech A, Toulopoulou T et al (2002) Brain volumes in familial and non familial schizophrenic probands and their unaffected relatives. Neuropsychiatric Genetics 114:616–625
Spitzer RL, Endicott J, Robins E. Research Diagnostic Criteria (RDC) for a selected group of functional disorders. New York State Psychiatric Institution, Biometrics Research Division, New York
Endicott J, Spitzer RL (1978) A diagnostic interview: the Schedule for Affective Disorders and Schizophrenia. Arch Gen Psychiatry 35:837–844
Endicott J, Andreasen NC, Spitzer RL (1978) Family history research diagnostic criteria. New York State Psychiatric Institution, New York
Nurnberger JI, Blehar MC, Kaufmann CA, York-Cooler C, Simpson SG, Harkavy-Friedman J, Severe JB, Malaspina D, Reich T (1994) Diagnostic interview for genetic studies. Rationale, unique features, and training. NIMH Genetics Initiative. Arch Gen Psychiatry 51:849–859
Barta PE, Dhingra L, Royall R, Schwartz E (1997) Improving stereological estimates for the volume of structures identified in three-dimensional arrays of spatial data. J Neurosci Methods 154:661–666
Duvernoy HM (1998) The human hippocampus: functional anatomy, vascularization and serial sections with MRI. Springer, Berlin Heidelberg New York
Bronen RA, Cheung G (1991) MRI of the temporal lobe: normal variations with special reference towards epilepsy. Magn Reson Imaging 9:501–507
Jackson GD, Berkovic SF, Duncan JS, Connelly A (1993) Optimizing the diagnosis of hippocampal sclerosis using MR imaging. AJNR Am J Neuroradiol 14:753–762
Baldwin GN, Tsuruda JS, Maravilla KR, Hamill GS, Hayes CE (1994) The fornix in patients with seizures caused by unilateral hippocampal sclerosis: detection of unilateral volume loss on MR images. AJR Am J Roentgenol 162:1185–1189
Sumi SM (1970) Brain malformations in the trisomy 18 syndrome. Brain 93:821–830
Kier EL, Kim JH, Fulbright RK, Bronen R (1997) Embryology of the human fetal hippocampus: MR imaging, anatomy, and histology. AJNR Am J Neuroradiol 18:525–532
Tsuang MT, Stone WS, Faraone SV (2001) Genes, environment and schizophrenia. Br J Psychiatry 178:s18–s24
Jones PB, Murray RM (1991) The genetics of schizophrenia is the genetics of neurodevelopment. Br J Psychiatry 158:615–623
Crow TJ, Ball J, Bloom SR et al (1989) Schizophrenia as an anomaly of development of cerebral asymmetry. A postmortem study and a proposal concerning the genetic basis of the disease. Arch Gen Psychiatry 46:1145–1150
McCarley RW, Wible CG, Frumin M, Hirayasu Y, Levitt JJ, Fischer IA, Shenton ME (1999) MRI anatomy of schizophrenia. Biol Psychiatry 45:1099–1119
Nelson MD, Saykin AJ, Flashman LA, Riordan HJ (1998) Hippocampal volume reduction in schizophrenia as assessed by magnetic resonance imaging. Arch Gen Psychiatry 55:433–440
Allin M, Murray R (2002) Schizophrenia: a neurodevelopmental or neurodegenerative disorder? Curr Opin Psychiatry 15:9–15
Bilder RM (2001) Schizophrenia as a neurodevelopmental disorder. Curr Opin Psychiatry 14:9–15
McDonald B, Highley JR, Walker MA, Herron BM, Cooper SJ, Esiri MM, Crow TJ (2000) Anomalous asymmetry of fusiform and parahippocampal gyrus gray matter in schizophrenia: a postmortem study. Am J Psychiatry 157:40–47
Altshuler LL, Casanova MF, Goldberg TE, Kleinman JE (1990) The hippocampus and parahippocampus in schizophrenic, suicide and control brains. Arch Gen Psychiatry 47:1029–1034
Bogerts B, Meertz E, Schonfeldt-Bausch R (1985) Basal ganglia and limbic system pathology in schizophrenia. A morphometric study of brain volume and shrinkage. Arch Gen Psychiatry 42:784–791
Casanova MF, Rothberg B (2002) Shape distortion of the hippocampus: a possible explanation of the pyramidal cell disarray reported in schizophrenia. Schizophr Res 5:19–24
Highley JR, Esiri MM, McDonald B, Cooper SJ, Crow TJ (1998) Temporal-lobe length is reduced, and gyral folding is increased in schizophrenia: a post mortem study. Schizophr Res 34:1–12
Cannon M, Caspi A, Moffitt TE et al (2002) Evidence for early childhood pan-developmental impairment specific to schizophreniform disorder: results from a longitudinal birth cohort. Arch Gen Psychiatry 59:449–457
Pearlson GD, Barta PE, Powers RE et al (1997) Medial and superior temporal gyral volumes and cerebral asymmetry in schizophrenia versus bipolar disorder. Biol Psychiatry 41:1–14
Weinberger DR, DeLisi LE, Neophytides AN, Wyatt RJ (1981) Familial aspects of CT scan abnormalities in chronic schizophrenic patients. Psychiatr Res 4:65–71
Suddath RL, Casanova MF, Goldberg TE, Daniel DG, Kelsoe JR, Weinberger D (1990) Anatomical abnormalities in the brains of monozygotic twins discordant for schizophrenia. N Engl J Med 332:789–794
Cannon TD, Mednick SA, Parnas J, Schulsinger F, Praestholm, Vestergaard A (1993) Developmental brain abnormalities in the offspring of schizophrenic mothers. Arch Gen Psychiatry 50:551–564
Essen DC van (1997) A tension based theory of morphogenesis and compact wiring in the central nervous system. Nature 385:313–318
Armstrong E, Schleicher A, Omran H, Curtis M, Zilles K (1995) The ontogeny of human gyrification. Cereb Cortex 5:56–63
Bronen RA, Cheung G (1991) MRI of the normal hippocampus. Magn Reson Imaging 9:497–500
Mamourian AC, Cho CH, Saykin AJ, Poppito NL (1998) Association between size of the lateral ventricle and asymmetry of the fornix in patients with temporal lobe epilepsy. AJNR Am J Neuroradiol 19:9–13
Meiners LC, van Gils A, Jansen GH (1994) Temporal lobe epilepsy: the various MR appearances of histologically proven mesial temporal sclerosis. AJNR Am J Neuroradiol 15:1547–1555
Galaburda AM, LeMay M, Kemper TL, Geschwind N (1978) Right-left asymmetries in the brain. Science 199:852–856
Crow T (1990) Temporal lobe asymmetries as the key to etiology of schizophrenia. Schizophr Bull 3:433–443
Bilder RM, Bogerts B, Ashtari M et al (1995) Anterior hippocampal volume reductions predict frontal lobe dysfunction in first episode schizophrenia. Schizophr Res 17:47–58
Geschwind N, Galaburda AM. Cerebral lateralization (1985) Biological mechanisms, associations, and pathology: a hypothesis and a program for research. Arch Neurol 42:428–459
Lewis S (1995) Secondary schizophrenia. In: Hirsch SR, Weinberger DR (eds) Schizophrenia. Blackwell Oxford
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Connor, S.E.J., Ng, V., McDonald, C. et al. A study of hippocampal shape anomaly in schizophrenia and in families multiply affected by schizophrenia or bipolar disorder. Neuroradiology 46, 523–534 (2004). https://doi.org/10.1007/s00234-004-1224-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-004-1224-0