Abstract
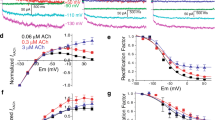
The effects of the agonist enantiomer S(−)Bay K 8644 on gating charge of L-type Ca channels were studied in single ventricular myocytes. From a holding potential (V h) of −40 mV, saturating (250 nM) S(−)Bay K shifted the half-distribution voltage of the activation charge (Q1) vs. V curve −7.5 ± 0.8 mV, almost identical to the shift produced in the Ba conductance vs. V curve (−7.7 ± 2 mV). The maximum Q1 was reduced by 1.7 ± 0.2 nC/µF, whereas Q2 (charge moved in inactivated channels) was increased in a similar amount (1.4 ± 0.4 nC/µF). The steady-state availability curves for Q1, Q2, and Ba current showed almost identical negative shifts of −14.8 ± 1.7 mV, −18.6 ± 5.8 mV, and −15.2 ± 2.7 mV, respectively. The effects of the antagonist enantiomer R(+)BayK 8644 were also studied, the Q1 vs. V curve was not significantly shifted, but Q1 max (V h = −40 mV) was reduced and the Q1 availability curve shifted by −24.6 ± 1.2 mV. We concluded that: a) the left shift in the Q1 vs. V activation curve produced by S(−)BayK is a purely agonistic effect; b) S(−)BayK induced a significantly larger negative shift in the availability curve than in the Q1 vs. V relation, consistent with a direct promotion of inactivation; c) as expected for a more potent antagonist, R(+)Bay K induced a significantly larger negative shift in the availability curve than did S(−) Bay K.









Similar content being viewed by others
Notes
1This protocol (also used by Shirokov et al., 1992) simplifies the separation between Q1 and Q2, as it allows non-inactivated channels to return to the resting closed state during the interpulse at −70 mV and it allows the dissection of the charge moved by VLCC (Q Ca) from that moved by Na channels (Q Na).
2In the model, Q1 and Q2 should vary by the same amount; the availability data presented in Fig. 3 is not conclusive in this regard. The observed difference could be explained by the variability of the steady-state level of inactivation in the absence of drug (note large error bars in the availability curve for Q1 Ca at saturating voltages) and because the measurements were done in different groups of cells. In any case, our qualitative conclusions about DHP’s actions are not affected by this variability because we always compared the situations in the absence and in the presence of drug in the same cell. Furthermore, we found the parallel shift in mid-voltage of both availability curves to strongly support the interconversion of the Q1 into the Q2 mechanism.
References
B.P. Bean (1984) ArticleTitleNitrendipine block of cardiac calcium channels: high-affinity binding to the inactivated state. Proc. Natl. Acad. Sci. USA 81 6388–6392 Occurrence Handle6093100
B.P. Bean E. Ríos (1989) ArticleTitleNonlinear charge movement in mammalian cardiac ventricular cells. Components from Na and Ca channel gating. J. Gen. Physiol. 94 65–93 Occurrence Handle2553859
M. Bechem S. Hebisch M. Schramm (1988) ArticleTitleCa2+ agonists: new sensitive probes for Ca2+ channels. Trends Pharmacol. Sci. 9 257–261 Occurrence Handle10.1016/0165-6147(88)90156-3
F. Bezanilla R.E. Taylor J.M. Fernandez (1982) ArticleTitleDistribution and kinetics of membrane dielectric polarization. I. Long-term inactivation of gating currents. J. Gen. Physiol. 79 21–40 Occurrence Handle7061986
G. Brum E. Ríos (1987) ArticleTitleIntramembrane charge movement in skeletal muscle fibers. Properties of charge 2. J. Physiol. 387 489–517 Occurrence Handle3116215
G. Brum R. Fitts G. Pizarro E. Ríos (1988) ArticleTitleVoltage sensors of the frog skeletal muscle membrane require calcium to function in excitation-contraction coupling. J. Physiol. 398 475–505 Occurrence Handle3260626
J.S. Fan Y. Yu P. Palade (2000) ArticleTitleKinetic effects of FPL 64176 on L-type Ca2+ channels in cardiac myocytes. Naunyn Schmiedebergs Arch. Pharmacol. 361 465–476 Occurrence Handle10.1007/s002100000219 Occurrence Handle10832599
G. Ferreira P. Artigas G. Pizarro G. Brum (1997) ArticleTitleButanedione monoxime promotes voltage-dependent inactivation of Ca channels in heart: Effects on gating currents. J. Mol. Cell. Cardiol. 29 777–787 Occurrence Handle10.1006/jmcc.1996.0321 Occurrence Handle1:CAS:528:DyaK2sXivVSgtbw%3D Occurrence Handle9140834
M. Grabner Z. Wang S. Hering J. Striessnig H. Glossman (1996) ArticleTitleTransfer of 1,4-dihydropyridine sensitivity from L-type to class A (BI) calcium channels. Neuron. 16 207–218 Occurrence Handle1:CAS:528:DyaK28XosFCqtw%3D%3D Occurrence Handle8562085
R. Hadley R.W. Lederer (1992) ArticleTitleComparison of the effects of Bay K 8644 on cardiac Ca2+ currents and Ca2+ channel gating current. Am. J. Physiol. 262 H472–H477 Occurrence Handle1:CAS:528:DyaK38Xhs1Kjsbw%3D Occurrence Handle1371650
R.W. Hadley J.R. Hume (1987) ArticleTitleAn intrinsic potential-dependent inactivation mechanism associated with calcium channels in guinea-pig myocytes. J. Physiol. 389 205–222 Occurrence Handle1:STN:280:BieD283hvFY%3D Occurrence Handle2445973
S.L. Hamilton A. Yatani K. Brusch A. Schwartz A.M. Brown (1987) ArticleTitleA comparison between the binding and electrophysiological effects of dihydropyridines on cardiac membranes. Mol. Pharmacol. 31 221–231 Occurrence Handle1:CAS:528:DyaL2sXitVOnsLo%3D Occurrence Handle2436031
O.P. Hamill A. Marty E. Neher B. Sakmann (1981) ArticleTitleImproved patch-clamp techniques for high resolution current recording from cells and cell free membrane patches. Pfluegers. Arch. 391 85–100 Occurrence Handle1:STN:280:Bi2D3sjhvVw%3D
S. Hering A.D. Hughes E.N. Timin T.B. Bolton (1993) ArticleTitleModulation of calcium channels in arterial smooth muscle cells by dihydropyridine enantiomers. J. Gen. Physiol. 101 393–410 Occurrence Handle1:CAS:528:DyaK3sXit1Ght7c%3D Occurrence Handle7682596
P. Hess J. Lansman R.W. Tsien (1984) ArticleTitleDifferent modes of Ca2+ channel gating behavior favored by dihydropyridine Ca agonist and antagonist. Nature 311 536–544
B. Hille (1977) ArticleTitleLocal anesthetics: hydrophilic and hydrophobic pathways for the drug-receptor reaction. J. Gen. Physiol. 69 497–515 Occurrence Handle1:CAS:528:DyaE2sXhvVejsLc%3D Occurrence Handle300786
I.R. Josephson N. Sperelakis (1990) ArticleTitleFast activation of cardiac Ca2+ channel gating charge by the dihydropyridine agonist BayK 8644. Biophys. J. 58 1307–1311 Occurrence Handle1:CAS:528:DyaK3cXmt1Cnsrs%3D Occurrence Handle1705451
T. Kamp M.C. Sanguinetti R.J. Miller (1989) ArticleTitleVoltage- and use-dependent modulation of cardiac calcium channels by dihydropyridine (+) 202–791. Circ. Research. 69 338–351
R.S. Kass (1987) ArticleTitleVoltage-dependent modulation of cardiac calcium channel current by optical isomers of Bay K 8644: Implications for channel gating. Circ. Research 61 11–15
A.E. Lacerda A.M. Brown (1989) ArticleTitleNon-modal gating of cardiac calcium channels as revealed by dihydropyridines. J. Gen. Physiol. 93 1243–1273 Occurrence Handle1:CAS:528:DyaL1MXkslGnurY%3D Occurrence Handle2475580
P. Lory F.A. Rassendren S. Richard F. Tiaho J. Nargeot (1990) ArticleTitleCharacterization of voltage-dependent calcium channels expressed in Xenopus oocytes injected with mRNA from rat heart. J. Physiol. 429 95–112 Occurrence Handle1:CAS:528:DyaK3MXit1Kmtb0%3D Occurrence Handle1703576
F. Markwardt B. Nilius (1988) ArticleTitleModulation of calcium channel currents in guinea-pig single ventricular heart cells by the dihydropyridine Bay K 8644. J. Physiol. 399 559–575 Occurrence Handle1:CAS:528:DyaL1cXht1Chtrc%3D Occurrence Handle2457095
T.F. Mc Donald S. Pelzer W. Trautwein D. Pelzer (1994) ArticleTitleRegulation and modulation of calcium channels in cardiac, skeletal and smooth muscle cells. Physiol. Rev. 74 365–507 Occurrence Handle1:CAS:528:DyaK2cXksl2jtLY%3D Occurrence Handle8171118
F. Nocetti R. Olcese N. Qin J. Zhou E. Stefani (1998) ArticleTitleEffect of Bay K 8644 (−) and the β2a subunit on Ca2+-dependent inactivation in α1c Ca2+ channels. J. Gen. Physiol. 111 463–471 Occurrence Handle10.1085/jgp.111.3.463 Occurrence Handle9482712
H. Reuter H. Porzig S. Kokubun B. Prod’hom (1988) ArticleTitleCalcium channels in the heart. Properties and modulation by dihydropyridine enantiomers. Ann. N. Y. Acad. Sci. 522 16–24 Occurrence Handle1:STN:280:BieB3sbjtFA%3D Occurrence Handle2454050
M.C. Sanguinetti R.S. Kass (1984) ArticleTitleVoltage-dependent block of calcium channel current in the calf cardiac Purkinje fiber by dihydropyridine calcium channel antagonists. Circ. Res. 55 336–348 Occurrence Handle1:CAS:528:DyaL2cXmtVCjtb4%3D Occurrence Handle6088117
M.C. Sanguinetti D.S. Krafte R.S. Kass (1986) ArticleTitleBay K8644: Voltage-dependent modulation of Ca channel current in heart cells. J. Gen. Physiol. 88 369–392 Occurrence Handle1:CAS:528:DyaL28Xls1Cksbo%3D Occurrence Handle2428922
R. Shirokov R. Levis N. Shirokova E. Ríos (1992) ArticleTitleTwo classes of gating charge from L-type calcium channels in guinea pig ventricular cells. J. Gen. Physiol. 99 863–895 Occurrence Handle1:STN:280:By2A2cbgs1E%3D Occurrence Handle1322450
X.Y. Wei A. Rutledge D.J. Triggle (1989) ArticleTitleVoltage-dependent binding of 1,4-dihydropyridine Ca2+ channel antagonist and activators in cultured neonatal rat ventricular myocytes. Mol. Pharmacology 35 541–552 Occurrence Handle1:CAS:528:DyaL1MXit1Ght78%3D
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
The four-states model proposed by Brum et al. (1988) for the voltage sensor of excitation contraction coupling of the skeletal muscle of the frog has been also used to reproduce some of the properties of the cardiac L-type Ca channel by Shirokov et al. (1992). This basic model (Scheme 1) consists of four states: closed (C), open (O) (admittedly an oversimplification as there is evidence for more than one closed state and the maximum P o of Ca channels is ~0.1 when all the activating charge has moved, a property not reproduced by this simple model), and two inactivated states (I o and I c) connected in a ring. The transitions between closed and open states and between inactivated states (horizontal transitions) are intrinsically voltage-dependent and generate charge 1 and charge 2, respectively. The transitions between available and inactivated states (vertical transitions) are assumed voltage-independent. An extension of this model assuming binding of DHP to all four states yields the eight-states model depicted in Scheme 2. Drug bound states are indicated by adding a D to the notation. The equilibrium constants between states are defined as follows:

The equilibrium between drug-free and drug-bound states is characterized by dissociation constants defined as K dO = O [D]/OD, K dC = C[D]/CD, K d1O = I O [D]/I O D, K d1C = I C [D]/I C D, where [D] is the DHP concentration.
Following Brum et al. (1988) K 1 = exp{(V−V 1)/k}, K 2 = exp{(V−V 2)/k}, K′1 = exp{(V−V′1)/k}, K′2 = exp{(V−V′2)/k}, where V 1 and V 2 are the half-distribution voltages for charge 1 and charge 2, respectively, in the drug-free form, and V′1 and V′2 in the drug-bound form. k is the e-fold voltage sensitivity, assumed to be the same for all the voltage-dependent steps. All these parameters of the model can be experimentally determined by the corresponding measurements in drug-free conditions and in the presence of saturating drug concentration.
As demonstrated by Brum et al. (1988), the four-state model steady-state availability curve is a Boltzmann function plus a constant. Its half distribution voltage, V 1/2, is given by

where k, the slope factor, has the same value as for Q1 and Q2 distributions. This equation is a reliable way to determine K 1, which can also be obtained as the ratio between the fraction of the total charge that inactivates and the fraction of the total charge that resists inactivation, provided that both measurements originate in the same set of channels.
The fourth equilibrium constant is not independent and is fixed by microscopic reversibility.
Similarly, the parameters for the drug-bound states were estimated in the presence of saturating drug concentration.

As microscopic reversibility applies to every possible cycle in the model, the twelve equilibrium constants are determined by five independent equations and only seven independent constants. As mentioned above, six of them were experimentally determined from the charge distributions and steady-state availability curves while the seventh is constrained by the dose-response curve. For instance,

Some general conclusions can be drawn, from (v) K dO/K dC = K I/K′1 = exp{(K′1−V 1)/k}<1 since V′1 < V 1 therefore, the dissociation constant of the open state is smaller than that of the closed state. From (iv) since K′2/K 2 = 1, based on the lack of change of the half distribution voltage of Q2 upon drug application, the drug should bind to both inactivated states with equal affinity. Finally from (iii) it is concluded that K dO/K d1O>1, since K′1/K 1>1, given the more negative mid-voltage of the availability curve of the drug-bound form. Thus, the inactivated states have higher affinity for the drug, consistent with the promotion of inactivation by agonist drugs.
To simulate the experimental data with the agonist Bay K we chose the following values for the constants: V 1 = 3 mV, V′1 = −5 mV, V 2 = V′2 = −90 mV, K 1 = 1.5, K′1 = 4, K dO = 20 nM. The chosen value for K dO is equal to the K 0.5 value obtained by Lacerda and Brown (1989) for S(−)Bay K in their study of tail currents in ventricular myocytes. With the values used, above calculations based on microscopic reversibility yield K dI = 8 nM and K dC = 34 nM. The outcome of such simulations, as shown in Fig. 7. A, B, C, were calculated at 250 nM Bay K, a condition in which all the channels are in the drug-bound form, at the V h indicated in the figure. In D, the dose-response curve was calculated at V h = −40 mV and the parameters plotted, ΔQ 1max and ΔV 1, were obtained by fitting a Boltzmann function to the simulated data. This rather simple model successfully reproduces the stationary data, suggesting the plausibility of modulated receptor binding of DHP to the L-type channel.
From (A1), (A2), (iii), (iv) and (v) it follows that the shift in the mid point of the steady-state availability curve, ΔV 1/2 = V′1/2 − V 1/2, is given by:

Equation A3 expresses the contribution of drug binding to the different states to the overall effect on the availability curve. On this basis the statement that promotion of the open state will only shift V 1/2 by the same amount as V 1 can be put in quantitatively. If this is the case, as in Sanguinetti et al. (1986), K I is the same for both drug-free and drug-bound forms and K dI = K dO. Thus ΔV 1/2 = k ln (K dO /K dC), which is the shift in V 1.
This model is also able to reproduce the effects of the antagonist (simulations not shown), by setting −2 mV as the shift in the activation curve and most important, K d0 = 10 K dI, a requirement to account for the ~30% reduction in Q1 max from V h = −40 mV and a shift of −25 mV in the availability curve.
Rights and permissions
About this article
Cite this article
Artigas, P., Ferreira, G., Reyes, N. et al. Effects of the Enantiomers of BayK 8644 on the Charge Movement of L-type Ca Channels in Guinea-pig Ventricular Myocytes . J. Membrane Biol. 193, 215–227 (2003). https://doi.org/10.1007/s00232-003-2020-1
Received:
Issue Date:
DOI: https://doi.org/10.1007/s00232-003-2020-1