Abstract
Objective: The absorption kinetics of paracetamol is dependent on gastric emptying and its measurement was proposed as a non-invasive method to estimate gastric emptying rate. The objective of this study was to evaluate the intraindividual variability of paracetamol absorption kinetics after a semi-solid meal.
Methods: The pharmacokinetics of paracetamol was studied on two occasions in 15 healthy volunteers without Helicobacter pylori antibodies. A 1-g dose of paracetamol was given as a solution together with a standardised semi-solid meal and the subjects stayed in the supine position.
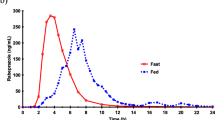
Results: For most of the subjects, the time course of paracetamol concentrations was similar on the two occasions. The intraindividual variability was low, with coefficients of variation of 38.3%, 8.0% and 3.8% for time to maximum plasma concentration, maximum concentration and area under the plasma concentration – time curve until 6 h, respectively.
Conclusion: The assessment of paracetamol absorption kinetics is reproducible when the drug is given together with a semi-solid meal in Helicobacter pylori-negative healthy subjects.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received: 21 July 1997 / Accepted in revised form: 14 October 1997
Rights and permissions
About this article
Cite this article
Paintaud, G., Thibault, P., Queneau, PE. et al. Intraindividual variability of paracetamol absorption kinetics after a semi-solid meal in healthy volunteers. E J Clin Pharmacol 53, 355–359 (1998). https://doi.org/10.1007/s002280050393
Issue Date:
DOI: https://doi.org/10.1007/s002280050393