Abstract
Purpose
Linezolid (LZD) levels are frequently insufficient in intensive care unit (ICU) patients receiving standard dose, which is predictive of a poor prognosis. Alternative dosing regimens are suggested to address these insufficient levels, which are substantial factors contributing to the emergence of multidrug-resistant bacteria, resulting in increased morbidity and mortality among people who are critically ill.
Methods
Forty-eight patients admitted to the intensive care unit were enrolled in an open-label, prospective, randomized study and assigned to one of three LZD administration modes: intermittent groupI (GpI) (600 mg/12 h), continuous infusion groupII (GpII) (1200 mg/24 h) or continuous infusion with loading dose groupIII (GpIII) (on Day 1, 300 mg intravenously plus 900 mg continuous infusion, followed by 1200 mg/24 h on Day 2). We evaluated serum levels of LZD using a validated ultra-performance liquid chromatography (UPLC) technique.
Results
Time spent with a drug concentration more than 85% over the minimum inhibitory concentration (T > MIC) was substantially more common in GpII and III than in GpI (P < 0.01). AUC/MIC values greater than 80 were obtained more frequently with continuous infusion GpIII and GpII than with intermittent infusion GpI, at 62.5%, 37.5% and 25%, respectively (P < 0.01). In GpI, the mortality rate was significantly higher than in the other groups.
Conclusion
In critically ill patients, continuous infusion with a loading dose (GpIII) is obviously superior to continuous infusion without a loading dose (GpII) or intermittent infusion (GpI) for infection therapy. Additionally, it might limit fluctuations in plasma concentrations, which may help overcome LZD resistance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Linezolid (LZD), the first oxazolidinone, is efficient against Gram-positive bacteria that are both susceptible and resistant to antibiotics, including methicillin-resistant Staphylococcus aureus (MRSA), multidrug-resistant Streptococcus pneumoniae and vancomycin-resistant Enterococci. This antibiotic is a key therapeutic choice for infections caused by Gram-positive bacterial pathogens that are resistant to other antibiotics in the intensive care unit (ICU). LZD is a medication that has been licensed for the treatment of community-acquired pneumonia, hospital-acquired infection and catheter-related bacteremia in a number of countries [1, 2].
Previous research has shown that LZD improves clinical features and microbiological outcomes in ICU patients. LZD-resistant organisms, on the other hand, have emerged, potentially leading to increased mortality and morbidity in ICU patients [3, 4].
To reduce the risk of underexposure to time-dependent antibiotics in the presence of difficult-to-treat infection, traditional administration has been replaced with a continuous infusion strategy [5]. The combination of pharmacokinetics (PK) and pharmacodynamics (PD) contributes to the optimization of the effectiveness of an antimicrobial agent. In addition, the rational use of PK and PD data enables an understanding of the effect of various dosage regimens on the time course of pharmacologic responses [6, 7]. Optimizing dosing regimens with the help of PK/PD data should also improve patient outcomes and prevent an increase in antimicrobial resistance. The most appropriate LZD’s PK/PD parameters are percentage of time that the drug concentration exceeds the minimum inhibitory concentration (MIC) (%T > MIC), area under the time–concentration curve to MIC (AUC/MIC) and the ratio of the maximum serum antibiotic concentration to MIC (Cmax/MIC) [1].
Patients who are critically ill are frequently at a greater risk of developing multidrug-resistant Gram-positive bacterial infections that are a significant public health concern. The majority of patients admitted to the ICU with bacteremia have significantly higher mortality rates than other patients. To put it another way, incorrect antimicrobial usage is a significant risk factor in the development of multidrug-resistant microorganisms, which increases morbidity and mortality. There was a higher success rate when T > MIC was more than 85% and AUC/MIC values ranged from 80 to 120 in studies of seriously ill adult patients [8, 9].
Our study aimed to compare three different modes of LZD administration in critically ill patients: intermittent (GPI), continuous infusion (GPII) and continuous infusion with a loading dosage (GpIII). In addition, to determine which is better for predicting clinical response and optimizing patient-specific therapy based on the PK/PD profile.
Subjects and method
This was a prospective, randomized, open-label trial carried out at Damanhour National Medical Institute’s ICU. The procedure was authorized by the Research Ethics Committee of Faculty of Pharmacy, Damanhour University (No. 1021PP40F). All patients provided written informed consent to participate in the study after being informed of the study’s aims and potential risks.
Subjects
Forty-eight Egyptian patients aged 23 to 87 years, weighing 93.65 kg and having a body mass index (BMI) between 25 and 42 kg/m2 were recruited from the ICU. The inclusion criteria included patients who were documented by Gram-positive pathogens sensitive to LZD therapy, fever (> 37.8 °C (100°F) and leukocytosis; white blood cells (WBCs) > 10,500 cells/mm3) were considered indicators of infection. The primary attending physician prescribed LZD to treat a bacterial organism that had been collected from a usually sterile place and was compatible with an infectious process.
Patients with any of the following criteria were excluded from participation: pregnancy, experienced a known adverse reaction to LZD in the past, platelet count < 80,000/mm3 or had a creatinine clearance (CrCl) < 40 ml/min which was estimated using the Cockcroft–Gault formula [10].
Study design
Participants were randomly allocated to one of three groups (n = 16) based on the recommended mode of LZD administration. All patients received the same total daily dose of 1200 mg. LZD was administered intravenously (IV) to GpI as a 30-min intermittent IV bolus (600 mg/12 h). LZD (1200 mg/24 h) was administered as a continuous infusion to GpII without previous loading dose administration. On the first day, LZD was given to GpIII as a 300 mg IV loading dosage is followed by a 900 mg continuous IV infusion, followed by a 1200 mg daily continuous infusion.
Medical records of patients assigned to the ICU revealed diagnostic data and data of their treatment plans. The Simplified Acute Physiology II Score (SAPS II) was used to predict mortality rates; WBCs and platelets were also evaluated. All participants in the study got recommended treatment for the infectious illness in accordance with recognized criteria. As clinically required, crystalloids and colloids were utilized to replenish fluids. Microbiological cultures were isolated from blood or any other possible source of infection before the first dosage of LZD.
Sample collection
Blood samples were taken from each patient treatment using an indwelling IV cannula implanted into the forearm’s antecubital vein; 10 mL was analyzed for complete blood cell count, urea, electrolytes, random blood glucose, liver function tests and renal function tests. Another 5 mL of blood sample was taken from each patient prior to LZD treatment.
The samples were collected in heparinized tubes at 2, 6, 12, 24, 36, 48, 60 and 72 h in patients from all groups (40 ml blood samples in total for each patient). At room temperature, blood samples were centrifuged at 4000 rpm for ten minutes, then plasma was collected over a period of six months and kept at – 80 °C until the LZD concentration was determined (during the time of samples collection) using a validated ultra-performance liquid chromatography (UPLC) technique. Additionally, urine samples for microscopic examination were taken.
Bioanalytic assessment of LZD
The quantities of LZD in serum were assessed using a validated UPLC technique. The mobile phase was composed of 0.2 percent phosphoric acid and acetonitrile in a 75:25 (vol/vol) ratio. The analysis was performed at a flow rate of 1.5 mL/min using an HPLC column Microsorb-MV 100–3 C18 (250 mm 4.6 mm, 5 mm; Agilent Technologies, the Netherlands) [5, 11, 12]. The effluent was monitored at a wavelength of 254 nm using an ultraviolet (UV) Agilent detector 1290 DAD (Model: G4212A; Serial No. DEBAF04676, USA) and an internal standard (IS; tinidazole). The peak areas of the drug and IS were electronically combined using an analysis program (Agilent Scientific Instruments) and the peak area ratio of the drug to IS was calculated.
The calibration standards for LZD were prepared by transferring 25 μL of each working solution and IS (tinidazole) to a set of test tubes. After evaporating the methanolic solvent, 0.25 mL blank plasma was added to each tube to create a set of calibration standards with concentrations of 1, 2, 5, 10, 20, 50 and 100 mg/L. After vortexing the sample, 0.4 mL acetonitrile was added, mixed for 2 min and then centrifuged for 6 min at 3000 rpm. After separating the supernatant, 20 μL was fed into the UPLC.
0.25 mL of each sample was transferred to a clean test tube for analysis. After adding the IS, the research samples were used as calibration standards (tinidazole). LZD and IS had a mean retention time of 4.33 ± 0.09 min and 3.50 ± 0.07 min, respectively, under the chromatographic conditions stated before.
LZD calibration curve linearity in human plasma was established using least-squares linear regression analysis of the peak area ratios of LZD to IS vs the corresponding LZD levels. Over the assay range (1–100 g/mL), the calibration curves were linear, with a correlation coefficient greater than 0.99. Within-day coefficient of variances (CVs) for LZD varied from 1.226 to 5.625%, but between-day CVs ranged from 2.371 to 6.680%.
PK analysis
LZD’s PK characteristics were determined as follows: AUC values were determined for the periods 0–24 h, 24–48 h and 48–72 h (AUC0–24, AUC24–48, AUC48–72), minimum and maximum concentrations of LZD at steady state (Cpss max, Cpss min), the elimination rate constant (Ke), the half-life (t1/2), clearance (CL) and volume of distribution (Vd). The PK parameters for LZD were determined using a non-compartmental model employing plasma drug concentration–time data. Ke for plasma concentration–time data points in the terminal log-linear area of the curves was determined using least-squares regression [7].
T1/2 was determined by dividing ln2 by Ke. The linear trapezoidal rule was used to determine the (AUC 0–t). The AUC from zero to infinity (AUC0–∞) was calculated as AUC0–∞ (AUC0–∞ + C/Ke). C denotes the last concentration determined Cmax and time to Cmax (i.e., Tmax) were determined directly from the concentration–time curves of individual plasma samples. Vd was calculated as D (1–e−Kt) divided by [ke t (Cmax–Cmin. e−Kt), where D is the dose, k is the elimination rate constant and t is the infusion time. The mean steady-state concentration is calculated as dosage divided by [Vd. Ke. DI]. CL value for LZD computed as (Ke. Vd) [6].
PK/PD model
MICs were established using broth microdilution [13]. The ratio of the maximal serum concentrations to the infecting organism’s MIC was calculated as (Cmax/MIC). The area under the concentration–time curve for a 24-h dosing interval relative to the organism’s minimal inhibitory concentration (AUC0–24/MIC) can be calculated quantitatively using the equation [14].
The amount of time during a dosing interval when the serum concentration is greater than the MIC (%T > MIC) was calculated analytically using the equation %T > MIC = ln [Dose/(Vd*MIC)]*[t1/2/0.693]*[100/DI] [15], where Vd denotes the apparent volume of distribution in the central compartment (L/kg), ke denotes the elimination rate constant (h−1), MIC is the minimal inhibitory concentration (mg/L) for the organism/antibiotic combination and DI denotes the dosing interval (h) [16].
Statistical analysis
The Minitab Statistical Package version 16 was used to conduct statistical comparisons between three approach treatments using a one-way analysis of variance model (Minitab, State College, Pennsyl Vania) [17]. A P value of ≤ 0.05 was taken as the level of significance.
Results
Baseline characteristics and study population
Forty-eight critically ill ICU patients were randomly allocated to one of three equal groups, (age range, 23–80 years; weight range, 70–110 kg; BMI range, 28.3–42.8 kg/m2) and all completed the trial. Patients’ demographic information were reported in (Table 1). There were no statistically significant differences in baseline characteristics between the three therapy groups. The average age and BMI of GpI, II, III participants were (56.5 ± 12.4 years, 33.17 ± 3.48 kg/m2), (59.11 ± 15.7 years, 32.96 ± 2.05 kg/m2) and (56 ± 17.63 years, 34.69 ± 3.31 kg/m2), respectively. GpI had a considerably higher mortality rate than the other groups. Eight patients died in GpI, five in GpII and just two in GpIII. The remaining patients experienced clinical success.
At admission, the most frequently encountered clinical diagnoses were hospital-acquired pneumonia (HAP) (n = 5 in GpI, n = 6 in GpII and n = 6 in GpIII), ventilator-acquired pneumonia (VAP) (n = 2 in GpI, n = 1 in GpII and n = 4 in GpIII), sepsis (n = 2 in GpI, n = 7 in GpII and n = 2 in GpIII) and septic shock (n = 4 in GpI, n = 3 in GpII). The mean length of LZD therapy was ten days (range, 7–15 days) across all groups.
Table 2 shows the location of microorganism isolation as well as the MIC of LZD. Staphylococci and Streptococci were the most frequently isolated pathogens. LZD’s MIC was 2 mg/L in 54% of isolates and 4 mg/L in 46% of isolates.
Pharmacokinetics
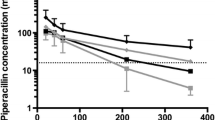
LZD’s mean serum concentration–time profiles were measured in the three groups of patients as shown in (Fig. 1). The major PK parameters of LZD were summarized in (Table 3). The serum Cmax of LZD was 12.1 ± 1.47 mg/L in GpI, with an average blood concentration of 11.33 ± 1.34 mg/L after 0.5 h. Following then, LZD’s mean plasma concentration rapidly decreased from 7.69 ± 1.93 to 4.93 ± 1.28 mg/L, eventually reaching 2.24 ± 0.56 mg/L (at 2, 6 and 12 h, respectively). The plasma concentration of LZD was determined every 12 h for 72 h following administration (Fig. 1). At 1, 2, 6 and 12 h, the mean serum LZD concentrations in GpII were 4.26 ± 0.85, 5.66 ± 1.04, 7.2 ± 1.43 and 8.65 ± 1.49 mg/L, respectively. In GpIII, the mean Cmax for total LZD was 14.2 ± 2.63 mg/L, with no significant difference from GpI. LZD concentrations at 1, 2, 6 and 12 h of continuous infusion were 11.76 ± 2.22, 11.32 ± 2.42, 10.68 ± 2.33 and 10.48 ± 2.47 mg/L, respectively. At 6 and 12 h, the latter two values were considerably greater than the GpI comparable trough concentrations (P < 0.01). This statistically significant difference in serum LZD concentrations remained constant throughout the experiment. The total trough concentrations after intermittent infusion (GpI) were substantially lower than the mean concentrations reported during continuous IV infusion in GpII and III (Fig. 1). There was no significant difference among the three groups in terms of CL, Vd, Ke or t1/2. However, the mean AUC values for LZD over the course of the research (3 days) were significantly different among the three groups (Table 4).
An evaluation of therapy efficacy based on PK and PD data
Following treatment, the likelihood of cure was determined in these patients by analyzing both clinical features and microbiological responses (Table 2). Additionally, the following PK/PD characteristics were included in the analysis as indicators of LZD efficacy: area under the curve (AUC0–24/MIC), highest serum antimicrobial level relative to the MIC (Cmax/MIC) and percentage of dosing intervals with a serum concentration higher than the MIC (% T/MIC) (Table 4).
Mean trough levels in GpI were primarily less than the susceptibility breakpoint (4 mg/L), whereas mean trough levels in GpII and GpIII were always greater than the susceptibility breakpoint (4 mg/L). AUC/MIC ratios larger than 80 were obtained in only 25% of all samples (GpI), 37.5% of all samples (GpII) and 62.5% of all samples (GpIII) (P < 0.05), indicating a greater intra-individual variation in GpI than in GpII or III. For GpI, II and III, the Cmax/average MIC values were 5.81, 3.22 and 3.12, respectively. For GpII and GpIII MICs of two mg/L and four mg/L, the mean %Tf > MIC was 100% as shown in (Table 4). However, the % Tf > MIC in GpI was 77.08% and 0% for two mg/L and four mg/L MICs, respectively. Clinically and microbiologically cured Group II and III patients with an AUC0–24/MIC > 80 and Tf% > MIC is 100% for MIC 2 and 4. By comparing the pharmacodynamics parameters such as AUC/MIC and T > MIC between each two groups using post hoc test, we found there was statistically significant difference between GpI and GpIII and between GpII and GpIII (Table 5). As a result, a statistically and clinically meaningful difference was discovered between GpII and GpIII.
Discussion
LZD is the first-line treatment for multidrug-resistant Staphylococcus aureus and vancomycin resistant Enterococcus. It is regarded as a primary therapy option for critically ill patients [18, 19].
Most studies on PK and PD have been done on healthy volunteers, but a few have been done in patients with specific illnesses [3, 20]. Furthermore, no comparison of the PK/PD markers for LZD in the most critically ill patients using traditional intermittent dosing with continuous infusion with or without loading doses has been made previously.
In critically ill patients who received 600 mg of LZD every 12 h, blood levels varied substantially, with low trough serum concentrations [21, 22]. This necessitates close monitoring of specific PK/PD features when LZD is administered to those patients [21, 23, 24].
In this research, the goal was to determine the most effective method of administering LZD intravenously in critically ill patients based on PK and PD studies. Additionally, A patient’s pathophysiological state is an important factor in influencing the likelihood of recovery.
LZD has a long duration of antibacterial activity due to its prolonged half-life, which is time-dependent. Due to the fact that it is a time-dependent antibiotic, AUC0–24/MIC and %T > MIC are frequently employed criteria for assessing its PD efficacy. It is most bactericidal when unbound concentrations of the medication above the bacterial pathogen’s (MIC) (%Tf > MIC).
LZD was most effective when the %T > MIC was > 85%, or when the AUC/MIC > 100. Additionally, Cmax/MIC is an excellent predictor of bactericidal activity, but because LZD is a time-dependent antibiotic, larger Cmax/MIC ratios may have little effect on the expected clinical outcome [22,23,24,25].
Our study shown that continuous infusion was capable of achieving AUC/MIC values between 80 and 120 and %T > MIC > 85%, resulting in a more favorable outcome with a lower mortality rate in those groups compared to intermittent treatment group. Additionally, more patients obtained %Tf > MIC values greater than 85% when LZD was given as a continuous infusion rather than intermittently.
PK/PD features are significantly altered in critically ill patients. As a result, providing the same dose of LZD to those patients frequently resulted in suboptimal plasma concentrations when compared to healthy participants, owing to the patients’ variations in drug clearance and volume of distribution [21, 23, 24].
LZD’s clearance and volume of distribution were slightly increased in our study when compared to previously published values for healthy individuals [26, 27]. Our findings indicated that continuous administration of LZD significantly reduced the observed substantial fluctuations in plasma levels reported with intermittent dosing of LZD. Thus, continuous administration provided a means of avoiding the poor dose observed in those critically ill patients.
The majority of patients improved clinically and were cured. Among the three treatment groups, there was a significant variation in the proportion of patients who improved, with a higher rate of improvement found in patients receiving LZD by continuous infusion (1200 mg/24 h). There were no adverse events seen in patients receiving LZD via continuous infusion compared to those receiving the same dose via intermittent infusion (600 mg/12 h).
Constant exposure of bacteria or pathogens to antimicrobials at concentrations close to the MIC is typically associated with the development of antimicrobial resistance [9, 28]. Resistance generated can be overcome by obtaining a plasma concentration greater than the MIC. Continuous infusion of LZD with or without a loading dose resulted in plasma concentrations greater than the MIC (2 or 4 mg/L) (%T > MIC = 100%). In comparison, intermittent dosing resulted in decreased plasma LZD concentrations and a failure to maintain above the MIC (T > MIC (4 mg/L) (LZD Breakpoint) = 0%) during the research period. This is another benefit of continuous infusion over intermittent infusion.
Patients with severely impaired renal function did not participate in the study which prevented us from extending the results to severe renal ICU patients, most patients received other antibiotics concomitantly, which may have influenced the clinical outcome, also restriction of PK analysis to the first 600 mg dose. All these conditions considered as the limitations of the study.
Consequently, further studies with ICU patients who have severe renal impairment are recommended to confirm the clinical benefits and safety of LZD’s continuous infusions in these populations.
Conclusion
LZD can be given continuously with or without a loading dose to treat infections in people who are very critically ill. Continuous infusions have great advantages over intermittent infusions for treating infections in these people. Additionally, continuous infusions might help maintain acceptable serum levels and limit fluctuations in plasma concentrations, which may help overcome LZD resistance, which is common in ICU patients. Continuous infusion with loading dose has a superior positive outcome clinically and statistically than continuous infusion without loading dose.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Adembri C, Fallani S, Cassetta MI et al (2008) Linezolid pharmacokinetic/pharmacodynamic profile in critically ill septic patients: intermittent versus continuous infusion. Int J Antimicrob Agents 31(2):122–129. https://doi.org/10.1016/j.ijantimicag.2007.09.009
Dong H, Xie J, Chen L et al (2014) Developments in the pharmacokinetic/pharmacodynamic index of linezolid: A step toward dose optimization using Monte Carlo simulation in critically ill patients. Int J Infect Dis 22:35–40. https://doi.org/10.1016/j.ijid.2014.01.016
Dong H, Wang X, Dong Y et al (2011) Clinical pharmacokinetic/pharmacodynamic profile of linezolid in severely ill Intensive Care Unit patients. Int J Antimicrob Agents 38(4):296–300. https://doi.org/10.1016/j.ijantimicag.2011.05.007
Davidson ID, Cobbold L (1996) The Single European Currency. Eur Monet Union Kingsdown Enq 14(6):245–249. https://doi.org/10.1007/978-1-349-24825-4_37
De Pascale G, Fortuna S, Tumbarello M et al (2015) Linezolid plasma and intrapulmonary concentrations in critically ill obese patients with ventilator-associated pneumonia: intermittent vs continuous administration. Intensive Care Med. https://doi.org/10.1007/s00134-014-3550-y
Helmy SA (2013) Pharmacokinetics and relative bioavailability evaluation of Linezolid suspension and tablet formulations. Drug Res (Stuttg) 63(9):489–494. https://doi.org/10.1055/s-0033-1347189
Conte JE, Golden JA, Kipps J, Zurlinden E (2002) Intrapulmonary pharmacokinetics of linezolid. Antimicrob Agents Chemother 46(5):1475–1480. https://doi.org/10.1128/AAC.46.5.1475-1480.2002
Barrasa H, Soraluce A, García EU et al (2020) Impact of Augmented Renal Clearance (ARC) on the Pharmacokinetics of Linezolid: Advantages of Continuous Infusion from a PK/PD Perspective. Vol 0. https://doi.org/10.1016/j.ijid.2020.02.044
Boak LM, Li J, Rayner CR, Nation RL (2007) Pharmacokinetic/pharmacodynamic factors influencing emergence of resistance to linezolid in an in vitro model. Antimicrob Agents Chemother 51(4):1287–1292. https://doi.org/10.1128/AAC.01194-06
Cockcroft DW, Gault H (1976) Cockcroft1976.Pdf. Nephron 16:31–41
Jacqueline C, Batard E, Perez L et al (2002) In vivo efficacy of continuous infusion versus intermittent dosing of linezolid compared to vancomycin in a methicillin-resistant Staphylococcus aureus rabbit endocarditis model. Antimicrob Agents Chemother 46(12):3706–3711. https://doi.org/10.1128/AAC.46.12.3706-3711.2002
Tobin CM (2001) A simple, isocratic high-performance liquid chromatography assay for linezolid in human serum. J Antimicrob Chemother 48(5):605–608. https://doi.org/10.1093/jac/48.5.605
Clinical and Laboratory Standards Institute (2020) Performance standards for antimicrobial susceptibility testing. 30th ed. CLSI supplement M100. Wayne, PA: Clinical and Laboratory Standards Institute
Mohr JF, Wanger A, Rex JH (2004) Pharmacokinetic/pharmacodynamic modeling can help guide targeted antimicrobial therapy for nosocomial gram-negative infections in critically ill patients. Diagn Microbiol Infect Dis 48(2):125–130. https://doi.org/10.1016/j.diagmicrobio.2003.09.010
Turnidge J (1998) What can be done about resistance to antibiotics? Br Med J 317(7159):645–647. https://doi.org/10.1136/bmj.317.7159.645
Evans W, Schentag J, Jusko W (1992) Title Applied Pharmacokinetics, 3rd Edition. Appl Ther
Diletti E, Hauschke D, Steinijans VW (1992) Sample size determination for bioequivalence assessment by means of confidence intervals. Int J Clin Pharmacol Ther Toxicol 30 Suppl 1:S51–8. http://europepmc.org/abstract/MED/1601532.
Wasserman S, Meintjes G, Maartens G (2016) Linezolid in the treatment of drug-resistant tuberculosis: the challenge of its narrow therapeutic index. Expert Rev Anti Infect Ther 14(10):901–915. https://doi.org/10.1080/14787210.2016.1225498
Takada H, Hifumi T, Nishimoto N et al (2017) Linezolid versus vancomycin for nosocomial pneumonia due to methicillin-resistant Staphylococcus aureus in the elderly: A retrospective cohort analysis: Effectiveness of linezolid in the elderly. Am J Emerg Med 35(2):245–248. https://doi.org/10.1016/j.ajem.2016.10.058
Dong H, Xie J, Wang T et al (2016) Pharmacokinetic/pharmacodynamic evaluation of linezolid for the treatment of staphylococcal infections in critically ill patients. Int J Antimicrob Agents 48(3):259–264. https://doi.org/10.1016/j.ijantimicag.2016.05.009
Joukhadar C, Frossard M, Mayer BX et al (2001) Impaired target site penetration of β-lactams may account for therapeutic failure in patients with septic shock. Crit Care Med 29(2):385–391. https://doi.org/10.1097/00003246-200102000-00030
Cai Y, Bai N, Liu X, Liang B, Wang J, Wang R (2015) Pharmacokinetic/pharmacodynamic research on three different infusion time regimens of linezolid in healthy Chinese volunteers. Int J Clin Pharmacol Ther. https://doi.org/10.5414/CP202317
Mehrotra R, De Gaudio R, Palazzo M (2004) Antibiotic pharmacokinetic and pharmacodynamic considerations in critical illness. Intensive Care Med 30(12):2145–2156. https://doi.org/10.1007/s00134-004-2428-9
Boselli E, Breilh D, Rimmelé T et al (2005) Pharmacokinetics and intrapulmonary concentrations of linezolid administered to critically ill patients with ventilator-associated pneumonia. Crit Care Med 33(7):1529–1533. https://doi.org/10.1097/01.CCM.0000168206.59873.80
Rayner CR, Forrest A, Meagher AK, Birmingham MC, Schentag JJ (2003) Clinical Pharmacodynamics of Linezolid in Seriously Ill Patients Treated in a Compassionate Use Programme. Clin Pharmacokinet 42(15):1411–1423. https://doi.org/10.2165/00003088-200342150-00007
Roger C, Roberts JA, Muller L (2018) Clinical Pharmacokinetics and Pharmacodynamics of Oxazolidinones. Clin Pharmacokinet 57(5):559–575. https://doi.org/10.1007/s40262-017-0601-x
Stalker DJ, Jungbluth GL, Hopkins NK, Batts DH (2003) Pharmacokinetics and tolerance of single- and multiple-dose oral and intravenous linezolid, an oxazolidinone antibiotic, in healthy volunteers. J Antimicrob Chemother 51(5):1239–1246. https://doi.org/10.1093/jac/dkg180
Levy SB, Bonnie M (2004) Antibacterial resistance worldwide: Causes, challenges and responses. Nat Med 10(12S):S122–S129. https://doi.org/10.1038/nm1145
Acknowledgements
The authors are grateful for the support of the healthcare professionals at Damanhour National Medical Institute for their participation and assistance in collecting the patient data, especially Dr. Ehab Hegazy
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). No sources of funding were used in the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
Soha El-Masry and Noha El-Khodary participated in research design and analyzed collected data statistically. Rasha El-Gaml collected participants’ clinical data and conducted experiments. Rania Abozahra contributed to design and interpretation of results. Ayman El-Tayar done eligibility evaluation and subjects’ enrollment. All authors participated in writing, revision and approval of the final copy of manuscript.
Corresponding author
Ethics declarations
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study or respective legal representatives.
Conflict of interest
No conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
El-Gaml, R.M., El-Khodary, N.M., Abozahra, R.R. et al. Applying pharmacokinetic/pharmacodynamic measurements for linezolid in critically ill patients: optimizing efficacy and reducing resistance occurrence. Eur J Clin Pharmacol 78, 1301–1310 (2022). https://doi.org/10.1007/s00228-022-03340-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-022-03340-z