Abstract
Purpose
Iberdomide is a cereblon E3 ligase modulator capable of redirecting the protein degradation machinery of the cell towards the elimination of target proteins potentially driving therapeutic effects. In vitro studies demonstrated that iberdomide predominantly undergoes oxidative metabolism mediated by cytochrome P450 (CYP) 3A4/5 but had no notable inhibition or induction of CYP enzymes. Consequently, the potential of iberdomide as a victim of drug-drug interactions (DDI) was evaluated in a clinical study with healthy subjects.
Methods
A total of 33 males and 5 females with 19 subjects per part were enrolled. Part 1 evaluated the pharmacokinetics (PK) of iberdomide alone (0.6 mg) and when administered with the CYP3A and P-gp inhibitor itraconazole (200 mg twice daily on day 1 and 200 once daily on days 2 through 9). Part 2 evaluated the PK of iberdomide alone (0.6 mg) and with CYP3A4 inducer rifampin (600 mg QD days 1 through 13). Plasma concentrations of iberdomide and the active metabolite M12 were determined by validated liquid chromatography-tandem mass spectrometry assay.
Results
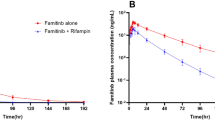
Coadministration of iberdomide with itraconazole increased iberdomide peak plasma concentration (Cmax) 17% and area under the concentration curve (AUC) approximately 2.4-fold relative to administration of iberdomide alone. The Cmax and AUC of iberdomide were reduced by approximately 70% and 82%, respectively, when iberdomide was administered with rifampin compared with iberdomide administered alone. Exploratory assessment of metabolite M12 concentrations demonstrated that CYP3A is responsible for M12 formation.
Conclusions
Caution should be taken when coadministering iberdomide with strong CYP3A inhibitors. Coadministration of iberdomide with strong CYP3A inducers is not advised.
Clinical trial registration
Clinical trial identification number is NCT02820935 and was registered in July 2016.




Similar content being viewed by others
References
Matyskiela ME, Zhang W, Man HW, Muller G, Khambatta G, Baculi F, Hickman M, LeBrun L, Pagarigan B, Carmel G, Lu CC, Lu G, Riley M, Satoh Y, Schafer P, Daniel TO, Carmichael J, Cathers BE, Chamberlain PP (2018) A cereblon modulator (CC-220) with improved degradation of Ikaros and Aiolos. J Med Chem 61(2):535–542
Chamberlain PP, Lopez-Girona A, Miller K, Carmel G, Pagarigan B, Chie-Leon B, Rychak E, Corral LG, Ren YJ, Wang M, Riley M, Delker SL, Ito T, Ando H, Mori T, Hirano Y, Handa H, Hakoshima T, Daniel TO, Cathers BE (2014) Structure of the human cereblon-DDB1-lenalidomide complex reveals basis for responsiveness to thalidomide analogs. Nat Struct Mol Biol 21(9):803–809
Gandhi AK, Kang J, Havens CG, Conklin T, Ning Y, Wu L, Ito T, Ando H, Waldman MF, Thakurta A, Klippel A, Handa H, Daniel TO, Schafer PH, Chopra R (2014) Immunomodulatory agents lenalidomide and pomalidomide co-stimulate T cells by inducing degradation of T cell repressors Ikaros and Aiolos via modulation of the E3 ubiquitin ligase complex CRL4(CRBN.). Br J Haematol 164(6):811–821
Lu G, Middleton RE, Sun H, Naniong M, Ott CJ, Mitsiades CS, Wong KK, Bradner JE, Kaelin WG (2014) The myeloma drug lenalidomide promotes the cereblon-dependent destruction of Ikaros proteins. Science 343(6168):305–309
Lonial S, v.d.D.N., Popat R, et al First clinical (phase 1b/2a) study of iberdomide (CC-220; IBER), a CELMoD, in combination with dexamethasone (DEX) in patients (pts) with relapsed/refractory multiple myeloma (RRMM) ASCO, 2019. #8006
Thomas M, Y. Y, Weiss D et al (2015) Evaluation of the safety, tolerability, pharmacokinetics (PK), and pharmacodynamics (PD) of multiple oral doses of CC-220 in healthy subjects. Clin Pharmacol Ther 97(Supplement 1):S22
LeCluyse EL (2001) Human hepatocyte culture systems for the in vitro evaluation of cytochrome P450 expression and regulation. Eur J Pharm Sci 13(4):343–368
Madan A, Graham RA, Carroll KM, Mudra DR, Burton LA, Krueger LA, Downey AD, Czerwinski M, Forster J, Ribadeneira MD, Gan LS, LeCluyse EL, Zech K, Robertson P Jr, Koch P, Antonian L, Wagner G, Yu L, Parkinson A (2003) Effects of prototypical microsomal enzyme inducers on cytochrome P450 expression in cultured human hepatocytes. Drug Metab Dispos 31(4):421–431
Bjornsson TD, Callaghan JT, Einolf HJ, Fischer V, Gan L, Grimm S, Kao J, King SP, Miwa G, Ni L, Kumar G, McLeod J, Obach RS, Roberts S, Roe A, Shah A, Snikeris F, Sullivan JT, Tweedie D, Vega JM, Walsh J, Wrighton SA (2003) The conduct of in vitro and in vivo drug-drug interaction studies: a Pharmaceutical Research and Manufacturers of America (PhRMA) perspective. Drug Metab Dispos 31(7):815–832
FDA (2006) Draft Guidance for Industry. https://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm292362.pdf
Ke AB, Zamek-Gliszczynski MJ, Higgins JW, Hall SD (2014) Itraconazole and clarithromycin as ketoconazole alternatives for clinical CYP3A inhibition studies. Clin Pharmacol Ther 95(5):473–476
Furie R, W. V, Gaudy A et al (2017) A randomized, placebo-controlled, double-blind, ascending-dose, safety, and pharmacokinetics study of CC-220 in subjects with systemic lupus erythematosus [abstract]. Arthritis Rheumatol 69(suppl 10)
Lonial S, A. M, Popat R et al (2019) Translational and Clinical evidence of a differentiated profile for the novel CELMoD, iberdomide (CC-220). Blood 134(Supplement_1)
Gaudy A, Y Y, Korish S et al (2017) SAT0225 Cereblon modulator CC-220 decreases naïve and memory B cells and plasmacytoid dendritic cells in systemic lupus erythematosus (SLE) patients: exposure-response results from a phase 2A proof of concept study. Ann Rheum Dis 76:858–859
Acknowledgments
The authors gratefully acknowledge Lisa Liu for the conduct of in vitro CYP inhibition studies described in this manuscript.
Data sharing
Data requests may be submitted to Celgene, A Bristol-Myers Squibb Company at https://vivli.org/ourmember/celgene/ and must include a description of the research proposal.
Funding
Support for this study and preparation of this manuscript was provided by Bristol-Myers Squibb Company.
Author information
Authors and Affiliations
Contributions
Allison Gaudy wrote the paper, analyzed PK data, and contributed to study design. Christian Atsriku analyzed the in vitro DDI studies data and contributed to study design. Ying Ye contributed to study design and study conduct. Kimberly MacGorman was the sponsor trial manager responsible for study design, conduct, and execution. Liangang Liu was the biostatistician responsible for statistical analysis. Yongjun Xue contributed to bioanalysis of the PK samples. Sekhar Surapaneni managed in vitro DDI studies and bioanalytical analysis. Maria Palmisano was the sponsor medical monitor for the trial.
Corresponding author
Ethics declarations
Conflict of interest
Allison Gaudy, Ying Ye, Kimberly MacGorman, Christian Atsriku, Liangang Liu, Yongjun Xue, Sekhar Surapaneni and Maria Palmisano are all full-time employees of Bristol Myers Squibb, and own stock in the company.
Code availability
Not applicable
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 36 kb)
Rights and permissions
About this article
Cite this article
Gaudy, A., Atsriku, C., Ye, Y. et al. Evaluation of iberdomide and cytochrome p450 drug-drug interaction potential in vitro and in a phase 1 study in healthy subjects. Eur J Clin Pharmacol 77, 223–231 (2021). https://doi.org/10.1007/s00228-020-03004-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-020-03004-w