Abstract
Purpose
To determine the steady state pharmacokinetics of trientine in children (≥ 12 years of age) and adult patients who had been receiving trientine dihydrochloride therapy prior to the study.
Methods
Twenty patients were exposed to trientine (trientine dihydrochloride capsules supplied by Univar) after standard oral dosing as part of ongoing therapy. Plasma trientine concentration was determined pre-dose and at 30 min, 1, 1.5, 2, 3, 4, 5, 6, 8, and 12 h post-dose. Concentrations of trientine in plasma were determined by LC-MS/MS using a validated bioanalytical method with stable labelled trientine as the internal standard.
Results
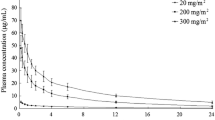
Trientine was generally absorbed fairly rapidly with a median Tmax of 1.49 h (range, 0.48–4.08 h). There was some variability in exposure, with a 10-fold range in Cmax, and a 13.8-fold range in AUC0-t. This variability was slightly lower when PK parameters were dose-normalised (6.7-fold range in Cmax/D and an 11.6-fold range in AUC0-t/D). The terminal half-life, which could be defined in 14 of the 20 patients, was broadly consistent between patients (range of 2.33 to 6.99 h). There was no marked difference in pharmacokinetics between adult patients (n = 16) and children (n = 4). The Cmax range was 506 to 3100 ng/mL in adults and 309 to 1940 ng/mL in children—the equivalent ranges for AUC0-t were 1240 to 17,100 ng/mL h and 1500 to 8060 ng/mL h. When PK parameters were normalised for administered dose, the Cmax/D and AUC0-t/D for children were contained within the ranges for the adult patients.
Conclusions
The steady state pharmacokinetics of trientine in Wilson disease patients were broadly similar to that reported in healthy subjects.

Similar content being viewed by others
References
Wilson S (1912) Progressive lenticular degeneration: a familial nervous disease associated with cirrhosis of the liver. Brain 34(4):295–509. https://doi.org/10.1093/brain/34.4.295
Ala A, Walker AP, Ashkan K, Dooley JS, Schilsky ML (2007) Wilson’s disease. Lancet 369(9559):397–408. https://doi.org/10.1016/S0140-6736(07)60196-2
Coffey AJ, Durkie M, Hague S, McLay K, Emmerson J, Lo C, Klaffke S, Joyce CJ, Dhawan A, Hadzic N, Mieli-Vergani G, Kirk R, Elizabeth Allen K, Nicholl D, Wong S, Griffiths W, Smithson S, Giffin N, Taha A, Connolly S, Gillett GT, Tanner S, Bonham J, Sharrack B, Palotie A, Rattray M, Dalton A, Bandmann O (2013) A genetic study of Wilson’s disease in the United Kingdom. Brain 136(Pt 5):1476–1487. https://doi.org/10.1093/brain/awt035
Bull PC, Thomas GR, Rommens JM, Forbes JR, Cox DW (1993) The Wilson disease gene is a putative copper transporting P-type ATPase similar to the Menkes gene. Nat Genet 5(4):327–337. https://doi.org/10.1038/ng1293-327
Tanzi RE, Petrukhin K, Chernov I, Pellequer JL, Wasco W, Ross B, Romano DM, Parano E, Pavone L, Brzustowicz LM, Devoto M, Peppercorn J, Bush AI, Sternlieb I, Pirastu M, Gusella JF, Evgrafov O, Penchaszadeh GK, Honig B, Edelman IS, Soares MB, Scheinberg IH, Gilliam TC (1993) The Wilson disease gene is a copper transporting ATPase with homology to the Menkes disease gene. Nat Genet 5(4):344–350. https://doi.org/10.1038/ng1293-344
Bandmann O, Weiss KH, Kaler SG (2015) Wilson’s disease and other neurological copper disorders. Lancet Neurol 14(1):103–113. https://doi.org/10.1016/S1474-4422(14)70190-5
Shah AB, Chernov I, Zhang HT, Ross BM, Das K, Lutsenko S, Parano E, Pavone L, Evgrafov O, Ivanova-Smolenskaya IA, Annerén G, Westermark K, Urrutia FH, Penchaszadeh GK, Sternlieb I, Scheinberg IH, Gilliam TC, Petrukhin K (1997) Identification and analysis of mutations in the Wilson disease gene (ATP7B): population frequencies, genotype-phenotype correlation, and functional analyses. Am J Hum Genet 61(2):317–328. https://doi.org/10.1086/514864
Thomas GR, Forbes JR, Roberts EA, Walshe JM, Cox DW (1995) The Wilson disease gene: spectrum of mutations and their consequences. Nat Genet 9(2):210–217. https://doi.org/10.1038/ng0295-210
Merle U, Schaefer M, Ferenci P, Stremmel W (2007) Clinical presentation, diagnosis and long-term outcome of Wilson’s disease: a cohort study. Gut 56(1):115–120. https://doi.org/10.1136/gut.2005.087262
Weiss KH. Wilson Disease. In: Pagon RA AM, Ardinger HH, Wallace SE, Amemiya A, Bean LJH, Bird TD, Ledbetter N, Mefford HC, Smith RJH, Stephens K, editors., SourceGeneReviews ® [Internet]. Seattle (WA): University of Washington S, 1993–2017., 29. OuJ, eds.1999
Ferenci P, Członkowska A, Merle U, Ferenc S, Gromadzka G, Yurdaydin C, Vogel W, Bruha R, Schmidt HT, Stremmel W (2007) Late-onset Wilson’s disease. Gastroenterology 132(4):1294–1298. https://doi.org/10.1053/j.gastro.2007.02.057
Ferenci P (2014) Phenotype-genotype correlations in patients with Wilson’s disease. Ann N Y Acad Sci 1315(1):1–5. https://doi.org/10.1111/nyas.12340
European Association for Study of the Liver (2012) EASL clinical practice guidelines: Wilson’s disease. J Hepatol 56(3):671–685. https://doi.org/10.1016/j.jhep.2011.11.007
Roberts EA, Schilsky ML (2008) (AASLD) AAfSoLD. Diagnosis and treatment of Wilson disease: an update. Hepatology 47(6):2089–2111. https://doi.org/10.1002/hep.22261
Weiss KH, Stremmel W (2014) Clinical considerations for an effective medical therapy in Wilson’s disease. Ann N Y Acad Sci 1315(1):81–85. https://doi.org/10.1111/nyas.12437
Tanabe R (1996) Disposition behavior and absorption mechanism of trientine, an orphan drug for Wilson’s disease. Hokkaido Igaky Zasshi 71(2):217–228
Cho HY, Blum RA, Sunderland T, Cooper GJ, Jusko WJ (2009) Pharmacokinetic and pharmacodynamic modeling of a copper-selective chelator (TETA) in healthy adults. J Clin Pharmacol 49(8):916–928. https://doi.org/10.1177/0091270009337939
Lu J, Poppitt SD, Othman AA, Sunderland T, Ruggiero K, Willett MS, Diamond LE, Garcia WD, Roesch BG, Cooper GJ (2010) Pharmacokinetics, pharmacodynamics, and metabolism of triethylenetetramine in healthy human participants: an open-label trial. J Clin Pharmacol 50(6):647–658. https://doi.org/10.1177/0091270009349379
Kobayashi M, Sugawara M, Saitoh H, Iseki K, Miyazaki K (1990) Intestinal absorption and urinary excretion of triethylenetetramine for Wilson’s disease in rat. Yakugaku Zasshi 110:759–763
Sakuma S, Tanno FK, Masaoka Y, Kataoka M, Kozaki T, Kamaguchi R, Kokubo H, Yamashita S (2007) Effect of administration site in the gastrointestinal tract on bioavailability of poorly absorbed drugs taken after a meal. J Control Release 118(1):59–64. https://doi.org/10.1016/j.jconrel.2006.12.016
Takeda S, Ono E, Matsuzaki Y, Wakui Y (1995) Metabolic fate of triethylenetetramine dihydrochloride (trientine hydrochloride, TJA-250) 3. Bioavailability of TJA-250 in rats after single administration. Pharmacometrics 49:179–186
Tanno FK, Sakuma S, Masaoka Y, Kataoka M, Kozaki T, Kamaguchi R, Ikeda Y, Kokubo H, Yamashita S (2008) Site-specific drug delivery to the middle-to-lower region of the small intestine reduces food-drug interactions that are responsible for low drug absorption in the fed state. J Pharm Sci 97:5341–5353
Acknowledgements
The authors wish to thank Johanna Braun, Christian Rupp, Daniel Gotthardt and Wolfgang Stremmel for their expertise and clinical contribution. The authors wish to acknowledge GfK for medical writing assistance.
Funding
This work was funded by Univar B.V. which had no influence on the results of the present study.
Author information
Authors and Affiliations
Contributions
JP designed and performed the study, discussed the results and contributed to the final manuscript.
CK discussed the results and contributed to the final manuscript.
PM analysed the samples, discussed the results and contributed to the final manuscript.
AH analysed the samples, discussed the results and contributed to the final manuscript.
KHW designed and performed the study, discussed the results and contributed to the final manuscript.
Corresponding author
Ethics declarations
The Ethics Committee of the Medical Faculty Heidelberg approved the study protocol and use of trientine in these patients. Informed consent was obtained from all patients prior to receiving any pre-study assessments.
Electronic supplementary material
Supplementary Figure 1
(DOCX 80 kb)
Supplementary Figure 2
(DOCX 154 kb)
Rights and permissions
About this article
Cite this article
Pfeiffenberger, J., Kruse, C., Mutch, P. et al. The steady state pharmacokinetics of trientine in Wilson disease patients. Eur J Clin Pharmacol 74, 731–736 (2018). https://doi.org/10.1007/s00228-018-2424-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-018-2424-6