Abstract
Purpose
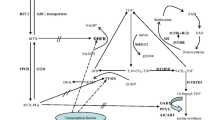
The antifolate drug methotrexate (MTX) was introduced into clinical practice about 60 years ago and remains an important component of different acute lymphoblastic leukemia (ALL) treatment protocols. It acts by inhibiting several enzymes in the folate pathway, thereby resulting in the disruption of folate homeostasis. To date, treatment regimens have not been personalized despite there being experimental evidence that gene polymorphisms of folate metabolizing enzymes affect MTX response. The aim of this review was to evaluate the influence of genetic polymorphisms of the enzymes involved in the MTX pathway on ALL treatment outcomes and identify factors underlining the failure to personalize MTX therapy.
Methods
We conducted a literature search in PUBMED and Goggle scholar using the following key words: methotrexate, polymorphism, acute lymphoblastic leukemia, pharmacogenetics, pharmacogenomics and personalized mediciner.
Results
The reasons for the failure to personalize MTX therapy may be due to (1) most studies involving single-center, small-sized cohorts, (2) differences in MTX dose across different protocols, (3) failure to consider minimal residual disease as a risk factor for post-induction treatment, (4) differences in outcome criteria between studies and (5) failure to consider the folate levels of a patient before initiation of MTX therapy. Although high-throughput techniques allow the mapping of thousands of genetic polymorphisms in a single run, it remains a major challenge to dissect out folate-metabolizing enzymes which have a high impact on the efficacy and toxicity of MTX and which, therefore, could be the targets for intervention.
Conclusions
Prospective pharmacogenetic studies which consider all of the above-mentioned factors should be undertaken to facilitate the design of personalized MTX treatment for ALL patients.

Similar content being viewed by others
Abbreviations
- MTX:
-
Methotrexate
- MTXPGs:
-
Methotrexate polyglutamates
- PCR:
-
Polymerase chain reaction
- MRD:
-
Minimal residual disease
- FISH:
-
Fluorescent in-situ hybridization
- LD:
-
Linkage disequilibrium
- EFS:
-
Event free survival
References
American Cancer Society. Cancer Facts & Figures 2011. Atlanta: American Cancer Society. Available from http://www.cancer.org/acs/groups/content@epidemiologysurveilance/documents/document/acspc-029771.
Kulkarni KP, Arora RS, Marwaha RK (2011) Survival outcome of childhood acute lymphoblastic leukemia in India: a resource-limited perspective of more than 40 years. J Pediatr Hematol Oncol 33:475–479. doi:10.1097/MPH.0b013e31820e7361
Schrappe M, Hunger SP, Pui C-H et al (2012) Outcomes after induction failure in childhood acute lymphoblastic leukemia. N Engl J Med 366:1371–1381. doi:10.1056/NEJMoa1110169
Smith M, Arthur D, Camitta B et al (1996) Uniform approach to risk classification and treatment assignment for children with acute lymphoblastic leukemia. J Clin Oncol Off J Am Soc Clin Oncol 14:18–24
Schultz KR, Pullen DJ, Sather HN et al (2007) Risk- and response-based classification of childhood B-precursor acute lymphoblastic leukemia: a combined analysis of prognostic markers from the Pediatric Oncology Group (POG) and Children’s Cancer Group (CCG). Blood 109:926–935. doi:10.1182/blood-2006-01-024729
Pui C-H, Sandlund JT, Pei D et al (2004) Improved outcome for children with acute lymphoblastic leukemia: results of Total Therapy Study XIIIB at St Jude Children’s Research Hospital. Blood 104:2690–2696. doi:10.1182/blood-2004-04-1616
Szczepański T, Orfão A, van der Velden VH et al (2001) Minimal residual disease in leukaemia patients. Lancet Oncol 2:409–417
Conter V, Bartram CR, Valsecchi MG et al (2010) Molecular response to treatment redefines all prognostic factors in children and adolescents with B-cell precursor acute lymphoblastic leukemia: results in 3184 patients of the AIEOP-BFM ALL 2000 study. Blood 115:3206–3214. doi:10.1182/blood-2009-10-248146
Borowitz MJ, Devidas M, Hunger SP et al (2008) Clinical significance of minimal residual disease in childhood acute lymphoblastic leukemia and its relationship to other prognostic factors: a Children’s Oncology Group study. Blood 111:5477–5485. doi:10.1182/blood-2008-01-132837
Estey EH, Appelbaum FR (eds) (2012) Leukemia and related disorders. Integrated treatment approaches series. Human Press, New York
Galivan J (1980) Evidence for the cytotoxic activity of polyglutamate derivatives of methotrexate. Mol Pharmacol 17:105–110
McGuire JJ, Bertino JR (1981) Enzymatic synthesis and function of folylpolyglutamates. Mol Cell Biochem 38 Spec No[Pt 1]:19–48
Genestier L, Paillot R, Quemeneur L et al (2000) Mechanisms of action of methotrexate. Immunopharmacology 47:247–257
Szeto DW, Cheng YC, Rosowsky A et al (1979) Human thymidylate synthetase—III. Effects of methotrexate and folate analogs. Biochem Pharmacol 28:2633–2637
Chan ESL, Cronstein BN (2002) Molecular action of methotrexate in inflammatory diseases. Arthritis Res 4:266–273
Schmiegelow K (2009) Advances in individual prediction of methotrexate toxicity: a review. Br J Haematol 146:489–503. doi:10.1111/j.1365-2141.2009.07765.x
Davidsen ML, Dalhoff K, Schmiegelow K (2008) Pharmacogenetics influence treatment efficacy in childhood acute lymphoblastic leukemia. J Pediatr Hematol Oncol 30:831–849. doi:10.1097/MPH.0b013e3181868570
Gorlick R, Goker E, Trippett T et al (1996) Intrinsic and acquired resistance to methotrexate in acute leukemia. N Engl J Med 335:1041–1048. doi:10.1056/NEJM199610033351408
Chango A, Emery-Fillon N, de Courcy GP et al (2000) A polymorphism (80G- > A) in the reduced folate carrier gene and its associations with folate status and homocysteinemia. Mol Genet Metab 70:310–315. doi:10.1006/mgme.2000.3034
Laverdière C, Chiasson S, Costea I et al (2002) Polymorphism G80A in the reduced folate carrier gene and its relationship to methotrexate plasma levels and outcome of childhood acute lymphoblastic leukemia. Blood 100:3832–3834. doi:10.1182/blood.V100.10.3832
Leyva-Vázquez MA, Organista-Nava J, Gómez-Gómez Y et al (2012) Polymorphism G80A in the reduced folate carrier gene and its relationship to survival and risk of relapse in acute lymphoblastic leukemia. J Investig Med Off Publ Am Fed Clin Res 60:1064–1067. doi:10.231/JIM.0b013e31826803c1
Shimasaki N, Mori T, Samejima H et al (2006) Effects of methylenetetrahydrofolate reductase and reduced folate carrier 1 polymorphisms on high-dose methotrexate-induced toxicities in children with acute lymphoblastic leukemia or lymphoma. J Pediatr Hematol Oncol 28:64–68. doi:10.1097/01.mph.0000198269.61948.90
Radtke S, Zolk O, Renner B et al (2013) Germline genetic variations in methotrexate candidate genes are associated with pharmacokinetics, toxicity, and outcome in childhood acute lymphoblastic leukemia. Blood 121:5145–5153. doi:10.1182/blood-2013-01-480335
Pakakasama S, Kanchanakamhaeng K, Kajanachumpol S et al (2007) Genetic polymorphisms of folate metabolic enzymes and toxicities of high dose methotrexate in children with acute lymphoblastic leukemia. Ann Hematol 86:609–611. doi:10.1007/s00277-007-0274-x
Goldman ID, Matherly LH (1985) The cellular pharmacology of methotrexate. Pharmacol Ther 28:77–102
Olsen EA (1991) The pharmacology of methotrexate. J Am Acad Dermatol 25:306–318
König J, Cui Y, Nies AT, Keppler D (2000) A novel human organic anion transporting polypeptide localized to the basolateral hepatocyte membrane. Am J Physiol Gastrointest Liver Physiol 278:G156–G164
Van de Steeg E, van der Kruijssen CMM, Wagenaar E et al (2009) Methotrexate pharmacokinetics in transgenic mice with liver-specific expression of human organic anion-transporting polypeptide 1B1 (SLCO1B1). Drug Metab Dispos Biol Fate Chem 37:277–281. doi:10.1124/dmd.108.024315
Treviño LR, Shimasaki N, Yang W et al (2009) Germline genetic variation in an organic anion transporter polypeptide associated with methotrexate pharmacokinetics and clinical effects. J Clin Oncol Off J Am Soc Clin Oncol 27:5972–5978. doi:10.1200/JCO.2008.20.4156
Ramsey LB, Panetta JC, Smith C et al (2013) Genome-wide study of methotrexate clearance replicates SLCO1B1. Blood 121:898–904. doi:10.1182/blood-2012-08-452839
Lopez-Lopez E, Martin-Guerrero I, Ballesteros J et al (2011) Polymorphisms of the SLCO1B1 gene predict methotrexate-related toxicity in childhood acute lymphoblastic leukemia. Pediatr Blood Cancer 57:612–619. doi:10.1002/pbc.23074
Danenberg PV (1977) Thymidylate synthetase - a target enzyme in cancer chemotherapy. Biochim Biophys Acta 473:73–92
Horie N, Aiba H, Oguro K et al (1995) Functional analysis and DNA polymorphism of the tandemly repeated sequences in the 5′-terminal regulatory region of the human gene for thymidylate synthase. Cell Struct Funct 20:191–197
Kaneda S, Nalbantoglu J, Takeishi K et al (1990) Structural and functional analysis of the human thymidylate synthase gene. J Biol Chem 265:20277–20284
Kawakami K, Salonga D, Park JM et al (2001) Different lengths of a polymorphic repeat sequence in the thymidylate synthase gene affect translational efficiency but not its gene expression. Clin Cancer Res Off J Am Assoc Cancer Res 7:4096–4101
Wang W, Marsh S, Cassidy J, McLeod HL (2001) Pharmacogenomic dissection of resistance to thymidylate synthase inhibitors. Cancer Res 61:5505–5510
Krajinovic M, Costea I, Chiasson S (2002) Polymorphism of the thymidylate synthase gene and outcome of acute lymphoblastic leukaemia. Lancet 359:1033–1034. doi:10.1016/S0140-6736(02)08065-0
Krajinovic M, Costea I, Primeau M et al (2005) Combining several polymorphisms of thymidylate synthase gene for pharmacogenetic analysis. Pharmacogenomics J 5:374–380. doi:10.1038/sj.tpj.6500332
Lauten M, Asgedom G, Welte K et al (2003) Thymidylate synthase gene polymorphism and its association with relapse in childhood B-cell precursor acute lymphoblastic leukemia. Haematologica 88:353–354
Pietrzyk JJ, Bik-Multanowski M, Skoczen S et al (2011) Polymorphism of the thymidylate synthase gene and risk of relapse in childhood ALL. Leuk Res 35:1464–1466. doi:10.1016/j.leukres.2011.04.007
Frosst P, Blom HJ, Milos R et al (1995) A candidate genetic risk factor for vascular disease: a common mutation in methylenetetrahydrofolate reductase. Nat Genet 10:111–113. doi:10.1038/ng0595-111
Weisberg I, Tran P, Christensen B et al (1998) A second genetic polymorphism in methylenetetrahydrofolate reductase (MTHFR) associated with decreased enzyme activity. Mol Genet Metab 64:169–172. doi:10.1006/mgme.1998.2714
Aplenc R, Thompson J, Han P et al (2005) Methylenetetrahydrofolate reductase polymorphisms and therapy response in pediatric acute lymphoblastic leukemia. Cancer Res 65:2482–2487. doi:10.1158/0008-5472.CAN-04-2606
Tanaka Y, Manabe A, Nakadate H et al (2013) Methylenetetrahydrofolate reductase gene haplotypes affect toxicity during maintenance therapy for childhood acute lymphoblastic leukemia in Japanese patients. Leuk Lymphoma. doi:10.3109/10428194.2013.825902
Chiusolo P, Reddiconto G, Farina G et al (2007) MTHFR polymorphisms’ influence on outcome and toxicity in acute lymphoblastic leukemia patients. Leuk Res 31:1669–1674. doi:10.1016/j.leukres.2007.03.028
Li W, Fan J, Hochhauser D et al (1995) Lack of functional retinoblastoma protein mediates increased resistance to antimetabolites in human sarcoma cell lines. Proc Natl Acad Sci USA 92:10436–10440
Hochhauser D, Schnieders B, Ercikan-Abali E et al (1996) Effect of cyclin D1 overexpression on drug sensitivity in a human fibrosarcoma cell line. J Natl Cancer Inst 88:1269–1275
Costea I, Moghrabi A, Krajinovic M (2003) The influence of cyclin D1 (CCND1) 870A > G polymorphism and CCND1-thymidylate synthase (TS) gene-gene interaction on the outcome of childhood acute lymphoblastic leukaemia. Pharmacogenetics 13:577–580. doi:10.1097/01.fpc.0000054123.14659.57
Costea I, Moghrabi A, Laverdiere C et al (2006) Folate cycle gene variants and chemotherapy toxicity in pediatric patients with acute lymphoblastic leukemia. Haematologica 91:1113–1116
Dulucq S, St-Onge G, Gagné V et al (2008) DNA variants in the dihydrofolate reductase gene and outcome in childhood ALL. Blood 111:3692–3700. doi:10.1182/blood-2007-09-110593
Al-Shakfa F, Dulucq S, Brukner I et al (2009) DNA variants in region for noncoding interfering transcript of dihydrofolate reductase gene and outcome in childhood acute lymphoblastic leukemia. Clin Cancer Res Off J Am Assoc Cancer Res 15:6931–6938. doi:10.1158/1078-0432.CCR-09-0641
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kodidela, S., Suresh Chandra, P. & Dubashi, B. Pharmacogenetics of methotrexate in acute lymphoblastic leukaemia: why still at the bench level?. Eur J Clin Pharmacol 70, 253–260 (2014). https://doi.org/10.1007/s00228-013-1623-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-013-1623-4