Abstract
Purpose
Cholinesterase inhibitors and memantine are the mainstay of pharmacological intervention for the cognitive symptoms of Alzheimer’s disease (AD). This study assessed the adequacy of dosing and persistence with AD medications and the predictors of these variables in the ‘real world’ (outside the clinical trial setting).
Methods
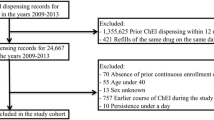
The Health Service Executive–Primary Care Reimbursement Services prescription claims database in the Republic of Ireland contains prescription information for 1.6 million people. Patients aged >70 years who received at least two prescriptions for donepezil, rivastigmine, galantamine and memantine between January 2006 and December 2010 were included in the study. Rates of dose-maximisation were recorded by examining the initiation dose of each AD drug commenced during the study period and any subsequent dose titrations. Non-persistence was defined by a gap in prescribing of more than 63 consecutive days. Predictors of dose-maximisation and non-persistence were also analysed.
Results
Between January 2006 and December 2010, 20,729 patients aged >70 years received a prescription for an AD medication. Despite most patients on donepezil and memantine receiving a prescription for the maximum drug dose, this dose was maintained for 2 consecutive months in only two-thirds of patients. Patients were significantly more likely to have their doses of donepezil and memantine maximised if prescribed in more recent years (2010 vs. 2007). Rates of non-persistence were 30.1 % at 6 months and 43.8 % at 12 months. Older age [75+ vs. <75 years; hazards ratio (HR) 1.16, 95 % confidence interval (CI) 1.06–1.27] and drug type (rivastigmine vs. donepezil; HR 1.15, 95 % CI 1.03–1.27) increased the risk of non-persistence. Non-persistence was lower for those commencing therapy in more recent years (2010 vs. 2007; HR 0.81, 95 % CI 0.73–0.89, p < 0.001) and for those on multiple anti-dementia medications (HR 0.59, 95 % CI 0.54–0.65, p < 0.001). Persistence was significantly higher when memantine was co-prescribed with donepezil (p < 0.0001).
Conclusion
Future studies should explore the reasons underlying non-persistence and failure to maintain dose-maximisation in patients on AD medications. There may be scope to improve the dosing and persistence with these medications in the community.


Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.References
Brookmeyer R, Johnson E, Ziegler-Graham K, Arrighi HM (2007) Forecasting the global burden of Alzheimer’s disease. Alzheimer’s Dementia 3:186–191
Lyketsos CG, Steinberg M, Tschanz JT, Norton MC, Steffens DC, Breitner JC (2000) Mental and behavioral disturbances in dementia: findings from the Cache County study on memory in aging. Am J Psychiatry 157:708–714
Tabet N (2006) Acetylcholinesterase inhibitors for Alzheimer’s disease: anti-inflammatories in acetylcholine clothing! Age Ageing 35(4):336–338
Finkel SI (2004) Effects of rivastigmine on behavioural and psychological symptoms of dementia in Alzheimer’s disease. Clin Ther 26:980–990
Birks J (2006) Cholinesterase inhibitors for Alzheimer’s Disease. Cochrane Database Syst Rev 2006 (1):CD005593
Takeda A, Loveman E, Clegg A et al (2006) A systematic review of the clinical effectiveness of donepezil, rivastigmine and galantamine on cognition, quality of life and adverse events in Alzheimer’s disease. Int J Geriatr Psychiatry 21:17–28
McShane R, Areosa SA, Minakaran N (2006) Memantine for dementia. Cochrane Database Syst Rev 2006 (2):CD003154
Grossberg GT (2008) Impact of rivastigmine on caregiver burden associated with Alzheimer’s disease in both informal care and nursing home settings. Drugs Aging 25:573–584
Doody RS, Geldmacher DS, Gordon B, Perdomo CA, Pratt RD (2001) Open-label, multicenter, phase 3 extension study of the safety and efficacy of donepezil in patients with Alzheimer disease. Arch Neurol 58:427–433
Raskind MA, Peskind ER, Truyen L, Kershaw P, Damaraju CV (2004) The cognitive benefits of galantamine are sustained for at least 36 months: a long-term extension trial. Arch Neurol 61:252–256
Farlow M, Anand R, Messina J, Hartman R, Veach J (2000) A 52-week study of the efficacy of rivastigmine in patients with mild to moderately severe Alzheimer’s disease. Eur Neurol 44:236–241
Howard R, McShane R, Lindesay J et al (2012) Donepezil and memantine for Moderate-to-Severe Alzheimer’s disease. N Engl J Med 366:893–903
electronic Medicines Compendium (eMC). Available at: http://www.medicines.org.uk/emc/
Andrade SE, Walker AM, Gottlieb LK et al (1995) Discontinuation of antihyperlipidemic drugs: do rates reported in clinical trials reflect rates in primary care setting? N Engl J Med 332(17):1125–1131
Suh DC, Thomas SK, Valiyeva E, Arcona S, Vo L (2005) Drug persistency of two cholinesterase inhibitors:rivastigmine versus donepezil in elderly patients with Alzheimer’s disease. Drugs Aging 22:695–707
Mucha L, Shaohung S, Cuffel B, McRae T, Mark TL, Del Valle M (2008) Comparison of cholinesterase inhibitor utilization patterns and associated health care costs in Alzheimer’s disease. J Manag Care Pharm 14:451–461
Thiruchselvam T, Naglie G, Moineddin R et al (2012) Risk factors for medication nonadherence in older adults with cognitive impairment who live alone. Int J Geriatr Psychiatry 27(12):1275–1282
Mauskopf JA, Paramore C, Lee WC, Snyder EH (2005) Drug persistency patterns for patients treated with rivastigmine or donepezil in usual care settings. J Mang Care Pharm 11:231–251
Barron TI, Connolly RM, Bennett K, Feely J, Kennedy MJ (2007) Early discontinuation of tamoxifen. A lesson for oncologists. Cancer 109:832–839
Perreault S, Blais L, Dragomir A et al (2005) Persistence and determinants of statin therapy among middle-ages patients free of cardiovascular disease. Eur J Clin Pharmacol 61:667–674
Irish Health Services Executive (HSE) Primary Care Reimbursement Services (PCRS) pharmacy-claims database. Available at: http://www.hse.ie/eng/Staff/PCRS/
Rogers SL, Farlow MR, Doody RS, Mohs R, Friedhoff LT (1998) A 24-week, double-blind, placebo-controlled trial of donepezil in patients with Alzheimer’s disease. Donepezil study group. Neurology 50:136–145
Rösler M, Anand R, Cicin-Sain A et al (1999) Efficacy and safety of rivastigmine in patients with Alzheimer’s disease: international randomised controlled trial. BMJ 318:633–638
Raskind MA, Peskind ER, Wessel T, Yuan W (2000) Galantamine in AD: A 6-month randomized, placebo-controlled trial with a 6-month extension. The galantamine USA-1 study group. Neurology 54:2261–2268
Tariot PN, Farlow MR, Grossberg GT, Graham SM, McDonald S, Gergel I, Memantine Study Group (2004) Memantine treatment in patients with moderate to severe Alzheimer disease already receiving donepezil: a randomized controlled trial. JAMA 291:317–324
Singh G, Thomas S, Vijayabharathi L (2005) Treatment and persistency with rivastigmine and donepezil in a large state Medicaid program. J Am Geriatr Soc 53:1269–1270
Blais L, Kergoat MJ (2009) Adherence to cholinesterase inhibitors in patients with alzheimers disease. J Am Geriatr Soc 57:366–368
Seltzer B (2007) Is long-term treatment of Alzheimer’s disease with cholinesterase inhibitor therapy justified? Drugs Aging 24:881–890
Balkrishnan R (1998) Predictors of medication adherence in the elderly. Clin Ther 20:764–771
Haynes RB, Ackloo E, Sahota N, McDonald HP, Yao X (2008) Interventions for enhancing medication adherence. Cochrane Database Syst Rev 2008 (2):CD000011
Oterberg L, Blaschke T (2005) Adherence to medication. N Engl J Med 353:487–497
National Institute for Health and Clinical Excellence (NICE) (2006) Dementia: Supporting people with dementia and their carers in health and social care. Clinical guidelines CG42. NICE, London
Lopez OL, Becker JT, Wahed AS et al (2009) Long-term effects of the concomitant use of memantine with cholinesterase inhibition in Alzheimer disease. J Neurol Neurosurg Psychiatry 80:600–607
Bushnell CD, Zimmer LO, Pan W, Adherence Evaluation After Ischemic Stroke–Longitudinal Investigators et al (2010) Persistence with stroke prevention medications 3 months after hospitalization. Arch Neurol 67(12):1456–1463
Alzheimer’s Research Trust (2010): Dementia 2010 Report. Alzheimer’s Research Trust UK, Cambridge
Rafii MS, Walsh S, Little JT et al (2011) Alzheimer’s Disease Cooperative Study. A phase II trial of huperzine A in mild to moderate Alzheimer disease. Neurology 76:1389–1394
Craft S, Baker LD, Montine TJ (2012) Intranasal insulin therapy for Alzheimer disease and amnestic mild cognitive impairment: a pilot clinical trial. Arch Neurol 69:29–38
Schrijvers EMC, Verhaaren BFJ, Koudstaal PJ et al (2012) Is dementia incidence declining? Trends in dementia incidence since 1990 in the Rotterdam study. Neurology 78:1456–1463
Li J, Wang YJ, Zhang M, Chongqing Ageing Study Group et al (2011) Vascular risk factors promote conversion from mild cognitive impairment to Alzheimer disease. Neurology 76(17):1485–1491
Acknowledgements
We would like to acknowledge the Primary Care Reimbursement Services from which this data was derived. The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Appendix 1
Appendix 1
Dosing and titration information recommended in specific product characteristics for medicines donepezil and memantine [13].
Medication | Dosing and titration recommendations |
|---|---|
Donepezil | Treatment is initiated at 5 mg/day. This dose should be maintained for at least 1 month in order to allow the earliest clinical responses to treatment to be assessed and to allow steady-state concentrations to be achieved. Following a 1-month clinical assessment of treatment at 5 mg/day, the dose can be increased to 10 mg/day (maximum recommended daily dose). |
Memantine | The recommended starting dose is 5 mg per day, which is increased over the first 4 weeks of treatment to reach the recommended maintenance dose of 20 mg daily. Week 1: One 5 mg film-coated tablet per day for 7 days. Week 2: One 10 mg film-coated tablet per day for 7 days. Week 3: One 15 mg film-coated tablet per day for 7 days. Week 4: One 20 mg film-coated tablet per day for 7 days. |
Rights and permissions
About this article
Cite this article
Brewer, L., Bennett, K., McGreevy, C. et al. A population-based study of dosing and persistence with anti-dementia medications. Eur J Clin Pharmacol 69, 1467–1475 (2013). https://doi.org/10.1007/s00228-013-1483-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-013-1483-y
Keywords
Profiles
- Kathleen Bennett View author profile