Abstract
Background
Benzimidazoles are often used concomitantly with protease inhibitors in patients with helminthic disease and HIV infection. Low bioavailability and extensive first-pass metabolism make benzimidazoles prone to pharmacokinetic drug interactions. The aim of the present study was to investigate potential drug interactions between the benzimidazoles albendazole and mebendazole and the potent CYP3A4 inhibitor ritonavir.
Methods
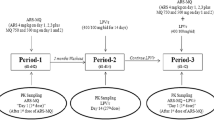
Sixteen healthy volunteers were administered a single oral dose of 1,000 mg mebendazole or 400 mg albendazole (2 × n = 8). AUC, Cmax, and t1/2 of mebendazole, albendazole, and albendazole sulfoxide were studied in absence and after short-term (2 doses) and long-term (8 days) treatment with ritonavir 200 mg bid.
Results
Pharmacokinetic parameters of albendazole and mebendazole were not changed by short-term administration of ritonavir. However, long-term administration of ritonavir resulted in significant changes in albendazole and mebendazole disposition, with a significant decrease in AUC0-24 (27 and 43% of baseline for albendazole and mebendazole, respectively) and Cmax (26 and 41% of baseline, respectively).
Conclusion
The AUC0-24 of benzimidazoles decreased after long-term use of ritonavir, while no changes in pharmacokinetic profiles were observed under short-term administration. These findings might help to optimize benzimidazole efficacy when used in combination with protease inhibitors.


Similar content being viewed by others
References
Ammann RW, Eckert J (1996) Cestodes. Echinococcus. Gastroenterol Clin North Am 25(3):655–689
Hosseinipour MC, Napravnik S, Joaki G, Gama S, Mbeye N, Banda B, Martinson F, Hoffman I, Cohen MS (2007) HIV and parasitic infection and the effect of treatment among adult outpatients in Malawi. J Infect Dis 195(9):1278–1282
Zingg W, Renner-Schneiter EC, Pauli-Magnus C, Renner EL, van Overbeck J, Schlapfer E, Weber M, Weber R, Opravil M, Gottstein B, Speck RF (2004) Alveolar echinococcosis of the liver in an adult with human immunodeficiency virus type-1 infection. Infection 32(5):299–302
Marriner SE, Morris DL, Dickson B, Bogan JA (1986) Pharmacokinetics of albendazole in man. Eur J Clin Pharmacol 30(6):705–708
Dawson M, Allan RJ, Watson TR (1982) The pharmacokinetics and bioavailability of mebendazole in man: a pilot study using [3H]-mebendazole. Br J Clin Pharmacol 14(3):453–455
Villaverde C, Alvarez AI, Redondo P, Voces J, Del Estal JL, Prieto JG (1995) Small intestinal sulphoxidation of albendazole. Xenobiotica 25(5):433–441
Rawden HC, Kokwaro GO, Ward SA, Edwards G (2000) Relative contribution of cytochromes P-450 and flavin-containing monoxygenases to the metabolism of albendazole by human liver microsomes. Br J Clin Pharmacol 49(4):313–322
Souhaili-El Amri H, Mothe O, Totis M, Masson C, Batt AM, Delatour P, Siest G (1988) Albendazole sulfonation by rat liver cytochrome P-450c. J Pharmacol Exp Ther 246(2):758–764
Merino G, Molina AJ, Garcia JL, Pulido MM, Prieto JG, Alvarez AI (2003) Intestinal elimination of albendazole sulfoxide: pharmacokinetic effects of inhibitors. Int J Pharm 263(1–2):123–132
Bekhti A, Pirotte J (1987) Cimetidine increases serum mebendazole concentrations. Implications for treatment of hepatic hydatid cysts. Br J Clin Pharmacol 24(3):390–392
Gottschall DW, Theodorides VJ, Wang R (1990) The metabolism of benzimidazole anthelmintics. Parasitol Today 6(4):115–124
Luder PJ, Siffert B, Witassek F, Meister F, Bircher J (1986) Treatment of hydatid disease with high oral doses of mebendazole. Long-term follow-up of plasma mebendazole levels and drug interactions. Eur J Clin Pharmacol 31(4):443–448
Ernest CS 2nd, Hall SD, Jones DR (2005) Mechanism-based inactivation of CYP3A by HIV protease inhibitors. J Pharmacol Exp Ther 312(2):583–591
Culm-Merdek KE, von Moltke LL, Gan L, Horan KA, Reynolds R, Harmatz JS, Court MH, Greenblatt DJ (2006) Effect of extended exposure to grapefruit juice on cytochrome P450 3A activity in humans: comparison with ritonavir. Clin Pharmacol Ther 79(3):243–254
Dixit V, Hariparsad N, Li F, Desai P, Thummel KE, Unadkat JD (2007) Cytochrome P450 enzymes and transporters induced by anti-HIV protease inhibitors in human hepatocytes: implications for predicting clinical drug interactions. Drug Metab Dispos 35:1853–1859
Yeh RF, Gaver VE, Patterson KB, Rezk NL, Baxter-Meheux F, Blake MJ, Eron JJ Jr, Klein CE, Rublein JC, Kashuba AD (2006) Lopinavir/ritonavir induces the hepatic activity of cytochrome P450 enzymes CYP2C9, CYP2C19, and CYP1A2 but inhibits the hepatic and intestinal activity of CYP3A as measured by a phenotyping drug cocktail in healthy volunteers. J Acquir Immune Defic Syndr 42(1):52–60
Wyen C, Fuhr U, Frank D, Aarnoutse R, Klaassen T, Lazar A, Seeringer A, Doroshyenko O, Kirchheiner J, Abdulrazik F, Schmeisser N, Lehmann C, Hein W, Schomig E, Burger D, Fatkenheuer G, Jetter A (2008) Effect of an antiretroviral regimen containing ritonavir boosted lopinavir on intestinal and hepatic CYP3A, CYP2D6 and P-glycoprotein in HIV-infected patients. Clin Pharmacol Ther 84:75–82
Mirfazaelian A, Dadashzadeh S, Rouini MR (2002) Effect of gender in the disposition of albendazole metabolites in humans. Eur J Clin Pharmacol 58(6):403–408
Hsu A, Granneman GR, Witt G, Locke C, Denissen J, Molla A, Valdes J, Smith J, Erdman K, Lyons N, Niu P, Decourt JP, Fourtillan JB, Girault J, Leonard JM (1997) Multiple-dose pharmacokinetics of ritonavir in human immunodeficiency virus-infected subjects. Antimicrob Agents Chemother 41(5):898–905
Fichtenbaum CJ, Gerber JG, Rosenkranz SL, Segal Y, Aberg JA, Blaschke T, Alston B, Fang F, Kosel B, Aweeka F (2002) Pharmacokinetic interactions between protease inhibitors and statins in HIV seronegative volunteers: ACTG Study A5047. Aids 16(4):569–577
FDA (2006) Guidance for industry. Drug interaction studies: study design, data analysis, and implications for dosing and labeling. FDA, Washington DC
Li XQ, Bjorkman A, Andersson TB, Gustafsson LL, Masimirembwa CM (2003) Identification of human cytochrome P(450)s that metabolise anti-parasitic drugs and predictions of in vivo drug hepatic clearance from in vitro data. Eur J Clin Pharmacol 59(5–6):429–442
Merino G, Jonker JW, Wagenaar E, Pulido MM, Molina AJ, Alvarez AI, Schinkel AH (2005) Transport of anthelmintic benzimidazole drugs by breast cancer resistance protein (BCRP/ABCG2). Drug Metab Dispos 33(5):614–618
Foisy MM, Yakiwchuk EM, Hughes CA (2008) Induction effects of ritonavir: implications for drug interactions. Ann Pharmacother 42(7):1048–1059
Smith CM, Faucette SR, Wang H, LeCluyse EL (2005) Modulation of UDP-glucuronosyltransferase 1A1 in primary human hepatocytes by prototypical inducers. J Biochem Mol Toxicol 19(2):96–108
van der Lee MJ, Dawood L, ter Hofstede HJ, de Graaff-Teulen MJ, van Ewijk-Beneken Kolmer EW, Caliskan-Yassen N, Koopmans PP, Burger DM (2006) Lopinavir/ritonavir reduces lamotrigine plasma concentrations in healthy subjects. Clin Pharmacol Ther 80(2):159–168
Schipper HG, Koopmans RP, Nagy J, Butter JJ, Kager PA, Van Boxtel CJ (2000) Effect of dose increase or cimetidine co-administration on albendazole bioavailability. Am J Trop Med Hyg 63(5–6):270–273
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Corti, N., Heck, A., Rentsch, K. et al. Effect of ritonavir on the pharmacokinetics of the benzimidazoles albendazole and mebendazole: an interaction study in healthy volunteers. Eur J Clin Pharmacol 65, 999–1006 (2009). https://doi.org/10.1007/s00228-009-0683-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-009-0683-y