Abstract
Objective
The objective of this study was to retrospectively evaluate the effects of MDR1, CYP3A4*18B, and CYP3A5*3 genetic polymorphisms on cyclosporine A (CsA) pharmacokinetics in Chinese renal transplant patients during the first month after transplantation.
Methods
A total of 103 renal transplant recipients receiving CsA were genotyped for MDR1 (C1236T, G2677T/A, and C3435T), CYP3A4*18B, and CYP3A5*3. The predose and 2-h postdose concentrations of CsA (C0 and C2, respectively) were determined by fluorescence polarization immunoassay, and their relationships with corresponding genotypes and haplotypes were investigated.
Results
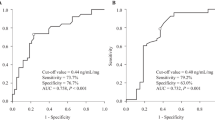
Patients with a CYP3A4*1/*1 genotype were found to have a higher dose-adjusted concentration compared with those with CYP3A4*18B/*18B, as follows: for C2, 19.3% (P = 0.008) during days 8-15, 35.2% (P = 0.008) during days 16–30, and for C0, 39.7% (P = 0.012) during days 16–30. The dose-adjusted C0 was higher in patients with MDR1 1236CC compared with those with 1236TT in the first month postoperation. The dose-adjusted C0 in patients with the CYP3A5*3/*3 genotype was 25.5% and 30.7% higher than those with the wild-type genotype during days 8–15 (P = 0.011) and days 16–30 (P = 0.015), respectively. Haplotype analysis revealed that the dose-adjusted C0 was higher in the first month following surgery in carriers of haplotype MDR1 CAC than in noncarriers. Polymorphisms of MDR1 and CYP3A5*3 did not affect dose-adjusted C2.
Conclusion
The data suggests that the CYP3A4*18B genotype affects CsA pharmacokinetics during the first month following surgery in Chinese renal transplant recipients. Patients with CYP3A4*18B alleles may require higher doses of CsA to reach the target levels. Large prospective studies may be needed to further explore the impact of MDR1 and CYP3A5*3 polymorphisms on CsA pharmacokinetics in renal transplant recipients.




Similar content being viewed by others
References
Hesselink DA, Smak Gregoor PJ, Weimar W (2004) The use of cyclosporine in renal transplantation. Transplant Proc 36(2 Suppl):99S–106S
Hebert MF (1997) Contributions of hepatic and intestinal metabolism and P-glycoprotein to cyclosporine and tacrolimus oral drug delivery. Adv Drug Deliv Rev 27(2–3):201–214
Kronbach T, Fischer V, Meyer UA (1988) Cyclosporine metabolism in human liver: identification of a cytochrome P-450III gene family as the major cyclosporine-metabolizing enzyme explains interactions of cyclosporine with other drugs. Clin Pharmacol Ther 43(6):630–635
Zhang Y, Benet LZ (2001) The gut as a barrier to drug absorption: combined role of cytochrome P450 3A and P-glycoprotein. Clin Pharmacokinet 40(3):159–168
Rendic S, Di Carlo FJ (1997) Human cytochrome P450 enzymes: a status report summarizing their reactions, substrates, inducers, and inhibitors. Drug Metab Rev 29(1–2):413–580
Cholerton S, Daly AK, Idle JR (1992) The role of individual human cytochromes P450 in drug metabolism and clinical response. Trends Pharmacol Sci 13(12):434–439
Kuehl P, Zhang J, Lin Y, Lamba J, Assem M, Schuetz J, Watkins PB, Daly A, Wrighton SA, Hall SD, Maurel P, Relling M, Brimer C, Yasuda K, Venkataramanan R, Strom S, Thummel K, Boguski MS, Schuetz E (2001) Sequence diversity in CYP3A promoters and characterization of the genetic basis of polymorphic CYP3A5 expression. Nat Genet 27(4):383–391
Fukushima-Uesaka H, Saito Y, Watanabe H, Shiseki K, Saeki M, Nakamura T, Kurose K, Sai K, Komamura K, Ueno K, Kamakura S, Kitakaze M, Hanai S, Nakajima T, Matsumoto K, Saito H, Goto Y, Kimura H, Katoh M, Sugai K, Minami N, Shirao K, Tamura T, Yamamoto N, Minami H, Ohtsu A, Yoshida T, Saijo N, Kitamura Y, Kamatani N, Ozawa S, Sawada J (2004) Haplotypes of CYP3A4 and their close linkage with CYP3A5 haplotypes in a Japanese population. Hum Mutat 23(1):100
Hu YF, Tu JH, Tan ZR, Liu ZQ, Zhou G, He J, Wang D, Zhou HH (2007) Association of CYP3A4*18B polymorphisms with the pharmacokinetics of cyclosporine in healthy subjects. Xenobiotica 37(3):315–327
Hall SD, Thummel KE, Watkins PB, Lown KS, Benet LZ, Paine MF, Mayo RR, Turgeon DK, Bailey DG, Fontana RJ, Wrighton SA (1999) Molecular and physical mechanisms of first-pass extraction. Drug Metab Dispos 27(2):161–166
Hoffmeyer S, Burk O, von Richter O, Arnold HP, Brockmoller J, Johne A, Cascorbi I, Gerloff T, Roots I, Eichelbaum M, Brinkmann U (2000) Functional polymorphisms of the human multidrug-resistance gene: multiple sequence variations and correlation of one allele with P-glycoprotein expression and activity in vivo. Proc Natl Acad Sci U S A 97(7):3473–3478
Cascorbi I, Gerloff T, Johne A, Meisel C, Hoffmeyer S, Schwab M, Schaeffeler E, Eichelbaum M, Brinkmann U, Roots I (2001) Frequency of single nucleotide polymorphisms in the P-glycoprotein drug transporter MDR1 gene in white subjects. Clin Pharmacol Ther 69(3):169–174
Kim RB, Leake BF, Choo EF, Dresser GK, Kubba SV, Schwarz UI, Taylor A, Xie HG, McKinsey J, Zhou S, Lan LB, Schuetz JD, Schuetz EG, Wilkinson GR (2001) Identification of functionally variant MDR1 alleles among European Americans and African Americans. Clin Pharmacol Ther 70(2):189–199
Yates CR, Zhang W, Song P, Li S, Gaber AO, Kotb M, Honaker MR, Alloway RR, Meibohm B (2003) The effect of CYP3A5 and MDR1 polymorphic expression on cyclosporine oral disposition in renal transplant patients. J Clin Pharmacol 43(6):555–564
von Ahsen N, Richter M, Grupp C, Ringe B, Oellerich M, Armstrong VW (2001) No influence of the MDR-1 C3435T polymorphism or a CYP3A4 promoter polymorphism (CYP3A4-V allele) on dose-adjusted cyclosporin A trough concentrations or rejection incidence in stable renal transplant recipients. Clin Chem 47(6):1048–1052
Mai I, Stormer E, Goldammer M, Johne A, Kruger H, Budde K, Roots I (2003) MDR1 haplotypes do not affect the steady-state pharmacokinetics of cyclosporine in renal transplant patients. J Clin Pharmacol 43(10):1101–1107
Chowbay B, Cumaraswamy S, Cheung YB, Zhou Q, Lee EJ (2003) Genetic polymorphisms in MDR1 and CYP3A4 genes in Asians and the influence of MDR1 haplotypes on cyclosporin disposition in heart transplant recipients. Pharmacogenetics 13(2):89–95
Hesselink DA, van Schaik RH, van der Heiden IP, van der Werf M, Gregoor PJ, Lindemans J, Weimar W, van Gelder T (2003) Genetic polymorphisms of the CYP3A4, CYP3A5, and MDR-1 genes and pharmacokinetics of the calcineurin inhibitors cyclosporine and tacrolimus. Clin Pharmacol Ther 74(3):245–254
Hu YF, Qiu W, Liu ZQ, Zhu LJ, Liu ZQ, Tu JH, Wang D, Li Z, He J, Zhong GP, Zhou G, Zhou HH (2006) Effects of genetic polymorphisms of CYP3A4, CYP3A5 and MDR1 on cyclosporine pharmacokinetics after renal transplantation. Clin Exp Pharmacol Physiol 33(11):1093–1098
Anglicheau D, Thervet E, Etienne I, Hurault De Ligny B, Le Meur Y, Touchard G, Buchler M, Laurent-Puig P, Tregouet D, Beaune P, Daly A, Legendre C, Marquet P (2004) CYP3A5 and MDR1 genetic polymorphisms and cyclosporine pharmacokinetics after renal transplantation. Clin Pharmacol Ther 75(5):422–433
Wang W, Zhang XD, Guan DL, Lu YP, Ma LL, Hu XP, Zhang P, Wang Y, Chen X (2005) Relationship between MDR1 polymorphism and blood concentration of cyclosporine A. Chin Med J (Engl) 118(24):2097–2100
Foote CJ, Greer W, Kiberd BA, Fraser A, Lawen J, Nashan B, Belitsky P (2006) MDR1 C3435T polymorphisms correlate with cyclosporine levels in de novo renal recipients. Transplant Proc 38(9):2847–2849
Haufroid V, Mourad M, Van Kerckhove V, Wawrzyniak J, De Meyer M, Eddour DC, Malaise J, Lison D, Squifflet JP, Wallemacq P (2004) The effect of CYP3A5 and MDR1 (ABCB1) polymorphisms on cyclosporine and tacrolimus dose requirements and trough blood levels in stable renal transplant patients. Pharmacogenetics 14(3):147–154
Jiao Z, Liang HQ, Ding JJ, Li ZD, Shi XJ, Zhong MK (2004) Effect of MDR1 polymorphic expression on oral disposition of cyclosporine A. Yao Xue Xue Bao 39(12):971–974
Johne A, Kopke K, Gerloff T, Mai I, Rietbrock S, Meisel C, Hoffmeyer S, Kerb R, Fromm MF, Brinkmann U, Eichelbaum M, Brockmoller J, Cascorbi I, Roots I (2002) Modulation of steady-state kinetics of digoxin by haplotypes of the P-glycoprotein MDR1 gene. Clin Pharmacol Ther 72(5):584–594
Anglicheau D, Verstuyft C, Laurent-Puig P, Becquemont L, Schlageter MH, Cassinat B, Beaune P, Legendre C, Thervet E (2003) Association of the multidrug resistance-1 gene single-nucleotide polymorphisms with the tacrolimus dose requirements in renal transplant recipients. J Am Soc Nephrol 14(7):1889–1896
Kuypers DR, de Jonge H, Naesens M, Lerut E, Verbeke K, Vanrenterghem Y (2007) CYP3A5 and CYP3A4 but not MDR1 single-nucleotide polymorphisms determine long-term tacrolimus disposition and drug-related nephrotoxicity in renal recipients. Clin Pharmacol Ther 82:711–725
Tang K, Ngoi SM, Gwee PC, Chua JM, Lee EJ, Chong SS, Lee CG (2002) Distinct haplotype profiles and strong linkage disequilibrium at the MDR1 multidrug transporter gene locus in three ethnic Asian populations. Pharmacogenetics 12(6):437–450
Chu XM, Hao HP, Wang GJ, Guo LQ, Min PQ (2006) Influence of CYP3A5 genetic polymorphism on cyclosporine A metabolism and elimination in Chinese renal transplant recipients. Acta Pharmacol Sin 27(11):1504–1508
Zhao Y, Song M, Guan D, Bi S, Meng J, Li Q, Wang W (2005) Genetic polymorphisms of CYP3A5 genes and concentration of the cyclosporine and tacrolimus. Transplant Proc 37(1):178–181
Levy G, Thervet E, Lake J, Uchida K (2002) Patient management by Neoral C(2) monitoring: an international consensus statement. Transplantation 73(9 Suppl):S12–18
Li D, Zhang GL, Lou YQ, Li Q, Wang X, Bu XY (2007) Genetic polymorphisms in MDR1 and CYP3A5 and MDR1 haplotype in mainland Chinese Han, Uygur and Kazakh ethnic groups. J Clin Pharm Ther 32(1):89–95
Kroetz DL, Pauli-Magnus C, Hodges LM, Huang CC, Kawamoto M, Johns SJ, Stryke D, Ferrin TE, DeYoung J, Taylor T, Carlson EJ, Herskowitz I, Giacomini KM, Clark AG (2003) Sequence diversity and haplotype structure in the human ABCB1 (MDR1, multidrug resistance transporter) gene. Pharmacogenetics 13(8):481–494
Aarnoudse AL, van Schaik RH, Dieleman J, Molokhia M, van Riemsdijk MM, Ligthelm RJ, Overbosch D, van der Heiden IP, Stricker BH (2006) MDR1 gene polymorphisms are associated with neuropsychiatric adverse effects of mefloquine. Clin Pharmacol Ther 80(4):367–374
Tanabe M, Ieiri I, Nagata N, Inoue K, Ito S, Kanamori Y, Takahashi M, Kurata Y, Kigawa J, Higuchi S, Terakawa N, Otsubo K (2001) Expression of P-glycoprotein in human placenta: relation to genetic polymorphism of the multidrug resistance (MDR)-1 gene. J Pharmacol Exp Ther 297(3):1137–1143
Fredericks S, Jorga A, MacPhee IA, Reboux S, Shiferaw E, Moreton M, Carter ND, Holt DW, Johnston A (2007) Multi-drug resistance gene-1 (MDR-1) haplotypes and the CYP3A5*1 genotype have no influence on ciclosporin dose requirements as assessed by C0 or C2 measurements. Clin Transplant 21(2):252–257
Sakaeda T, Nakamura T, Okumura K (2004) Pharmacogenetics of drug transporters and its impact on the pharmacotherapy. Curr Top Med Chem 4(13):1385–1398
Knight SR, Morris PJ (2007) The clinical benefits of cyclosporine C2-level monitoring: a systematic review. Transplantation 83(12):1525–1535
Pape L, Lehnhardt A, Latta K, Ehrich JH, Offner G (2003) Cyclosporin A monitoring by 2-h levels: preliminary target levels in stable pediatric kidney transplant recipients. Clin Transplant 17(6):546–548
Teisseyre J, Markiewicz M, Drewniak T, Kalicinski P, Kaminski A, Ismail H, Szymczak M, Teisseyre M, Nachulewicz P (2003) Switching cyclosporine blood concentration monitoring from C0 to C2 in children late after liver transplantation. Transplant Proc 35(6):2287–2288
Marcen R, Pascual J, Tato A, Villafruela JJ, Teruel JL, Rivera ME, Tenorio M, Fernandez M, Burgos FJ, Ortuno J (2003) Comparison of C0 and C2 cyclosporine monitoring in long-term renal transplant recipients. Transplant Proc 35(5):1780–1782
Acknowledgements
The authors thank Dr. Mingxin Li, Dr. Jianyong Zhong, and the staff of the renal transplantation center for their assistance; Mr. Yinqing Ying for his contribution in data input; Ms. Kitty Law for her critical reading of the manuscript; and Mr. Jianping Chen for statistical analysis support. This work was partly supported by the 100 Elites Program Grant of Shanghai Health Bureau (No 98BR009).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Qiu, Xy., Jiao, Z., Zhang, M. et al. Association of MDR1, CYP3A4*18B, and CYP3A5*3 polymorphisms with cyclosporine pharmacokinetics in Chinese renal transplant recipients. Eur J Clin Pharmacol 64, 1069–1084 (2008). https://doi.org/10.1007/s00228-008-0520-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-008-0520-8