Abstract
Objective
Leflunomide is a disease-modifying antirheumatic drug used for treating rheumatoid arthritis (RA). In vitro studies demonstrated that cytochromes P450 (CYPs), mainly CYP1A2 and CYP2C19, might be involved in leflunomide activation. The aim of our study was to investigate whether genetic polymorphisms of CYP1A2, CYP2C19, and CYP2C9 influence leflunomide toxicity.
Methods
A genotyping approach was used to determine CYP1A2*1F, CYP2C19*2, CYP2C19*17, CYP2C9*2, and CYP2C9*3 alleles in 105 RA patients.
Results
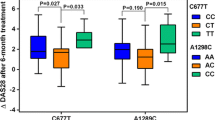
Leflunomide treatment was well tolerated by 62 patients, whereas 43 patients discontinued the treatment within the first year due to toxicity. Patients with CYP1A2*1F CC genotype had a 9.7-fold higher risk for overall leflunomide-induced toxicity than did the carriers of CYP1A2*1F A allele [P = 0.002, odds ratio = 9.708, 95% confidence interval = 2.276–41.403]. No significant association between the CYP2C19 and CYP2C9 genotypes and the leflunomide toxicity was observed.
Conclusion
Our results suggest that the CYP1A2*1F allele may be associated with leflunomide toxicity in RA patients.
Similar content being viewed by others
References
Mladenovic V, Domljan Z, Rozman B, Jajic I, Mihajlovic D, Dordevic J et al (1995) Safety and effectiveness of leflunomide in the treatment of patients with active rheumatoid arthritis. Results of a randomized, placebo-controlled, phase II study. Arthritis Rheum 38:1595–1603
Kalgutkar AS, Nguyen HT, Vaz AD, Doan A, Dalvie DK, McLeod DG et al (2003) In vitro metabolism studies on the isoxazole ring scission in the anti-inflammatory agent lefluonomide to its active alpha-cyanoenol metabolite A771726: mechanistic similarities with the cytochrome P450-catalyzed dehydration of aldoximes. Drug Metab Dispos 31:1240–1250
Sachse C, Brockmoller J, Bauer S, Roots I (1999) Functional significance of a C->A polymorphism in intron 1 of the cytochrome P450 CYP1A2 gene tested with caffeine. Br J Clin Pharmacol 47:445–449
De Morais SM, Wilkinson GR, Blaisdell J, Meyer UA, Nakamura K, Goldstein JA (1994) Identification of a new genetic defect responsible for the polymorphism of (S)-mephenytoin metabolism in Japanese. Mol Pharmacol 46:594–598
de Morais SM, Wilkinson GR, Blaisdell J, Nakamura K, Meyer UA, Goldstein JA (1994) The major genetic defect responsible for the polymorphism of S-mephenytoin metabolism in humans. J Biol Chem 269:15419–15422
Sim SC, Risinger C, Dahl ML, Aklillu E, Christensen M, Bertilsson L et al (2006) A common novel CYP2C19 gene variant causes ultrarapid drug metabolism relevant for the drug response to proton pump inhibitors and antidepressants. Clin Pharmacol Ther 79:103–113
He P, Court MH, Greenblatt DJ, Von Moltke LL (2005) Genotype-phenotype associations of cytochrome P450 3A4 and 3A5 polymorphism with midazolam clearance in vivo. Clin Pharmacol Ther 77:373–387
Chonlahan J, Halloran MA, Hammonds A (2006) Leflunomide and warfarin interaction: case report and review of the literature. Pharmacotherapy 26:868–871
Lim V, Pande I (2002) Leflunomide can potentiate the anticoagulant effect of warfarin. BMJ 325:1333
Sevilla-Mantilla C, Ortega L, Agundez JA, Fernandez-Gutierrez B, Ladero JM, Diaz-Rubio M (2004) Leflunomide-induced acute hepatitis. Dig Liver Dis 36:82–84
Rettie AE, Wienkers LC, Gonzalez FJ, Trager WF, Korzekwa KR (1994) Impaired (S)-warfarin metabolism catalysed by the R144C allelic variant of CYP2C9. Pharmacogenetics 4:39–42
Sullivan-Klose TH, Ghanayem BI, Bell DA, Zhang ZY, Kaminsky LS, Shenfield GM et al (1996) The role of the CYP2C9-Leu359 allelic variant in the tolbutamide polymorphism. Pharmacogenetics 6:341–349
Kunkel G, Cannon G (2006) Leflunomide in the treatment of rheumatoid arthritis. Expert Rev Clin Immunol 2:17–31
Aletaha D, Stamm T, Kapral T, Eberl G, Grisar J, Machold KP et al (2003) Survival and effectiveness of leflunomide compared with methotrexate and sulfasalazine in rheumatoid arthritis: a matched observational study. Ann Rheum Dis 62:944–951
van Roon EN, Jansen TL, van de Laar MA, Janssen M, Yska JP, Keuper R et al (2005) Therapeutic drug monitoring of A77 1726, the active metabolite of leflunomide: serum concentrations predict response to treatment in patients with rheumatoid arthritis. Ann Rheum Dis 64:569–574
Chan V, Charles BG, Tett SE (2005) Population pharmacokinetics and association between A77 1726 plasma concentrations and disease activity measures following administration of leflunomide to people with rheumatoid arthritis. Br J Clin Pharmacol 60:257–264
Li EK, Tam LS, Tomlinson B (2004) Leflunomide in the treatment of rheumatoid arthritis. Clin Ther 26:447–459
Rozman B (2002) Clinical pharmacokinetics of leflunomide. Clin Pharmacokinet 41:421–430
Smolen JS, Kalden JR, Scott DL, Rozman B, Kvien TK, Larsen A et al (1999) Efficacy and safety of leflunomide compared with placebo and sulphasalazine in active rheumatoid arthritis: a double-blind, randomised, multicentre trial. European Leflunomide Study Group. Lancet 353:259–266
Tiilikainen A, Fischer G, Grubic Z, Gyodi E, Ivaskova E, Jungerman M et al (1997) Anthropological features of the East European region. In: Charron D (ed) HLA: genetic diversity of HLA functional and medical implication. Proceedings of the 12th International Histocompatibility Workshop and Conference, EDK, Medical and Scientific International Publisher, Paris
Vidan-Jeras B, Jurca B, Dolzan V, Jeras M, Breskvar K, Bohinjec M (1998) Caucasian Slovenian normal. In: Gjertson D, Terasaki P (eds) HLA 1998, American society for histocompatibility and immunogenetics, Lenexa
Herman D, Dolzan V, Breskvar K (2003) Genetic polymorphism of cytochromes P450 2C9 and 2C19 in Slovenian population. Zdrav Vestn 72:347–351
van Roon EN, Yska JP, Raemaekers J, Jansen TL, van Wanrooy M, Brouwers JR (2004) A rapid and simple determination of A77 1726 in human serum by high-performance liquid chromatography and its application for optimization of leflunomide therapy. J Pharm Biomed Anal 36:17–22
Han XM, Ouyang DS, Chen XP, Shu Y, Jiang CH, Tan ZR et al (2002) Inducibility of CYP1A2 by omeprazole in vivo related to the genetic polymorphism of CYP1A2. Br J Clin Pharmacol 54:540–543
Rost KL, Roots I (1994) Accelerated caffeine metabolism after omeprazole treatment is indicated by urinary metabolite ratios: coincidence with plasma clearance and breath test. Clin Pharmacol Ther 55:402–411
Ghotbi R, Christensen M, Roh HK, Ingelman-Sundberg M, Aklillu E, Bertilsson L (2007) Comparisons of CYP1A2 genetic polymorphisms, enzyme activity and the genotype-phenotype relationship in Swedes and Koreans. Eur J Clin Pharmacol 63:537–546
Acknowledgements
The authors thank Magnus Ingelman-Sundberg and Sarah Sim from Karolinska Institute, Stockholm, Sweden, for kindly providing control DNA samples with known CYP2C19 *17 genotype.
We acknowledge Mojca Kos-Golja, M.D., Sonja Praprotnik, M.D., Ph.D., Aleš Ambrožič, M.D., Ph.D., Cvetka Kastelic-Klasinc, M.D., and Boris Lestan, M.D., M.Sc. from the Department of Rheumatology, University Medical Centre Ljubljana, Slovenia, for referring the patients. We thank Milena Pavić-Nikolić for her excellent technical support. We are especially grateful to Snežna Sodin-Šemrl, M.S., Ph.D. for language corrections. This work was financially supported by The Slovenian Research Agency, grant no. PO-0503–0381. The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bohanec Grabar, P., Rozman, B., Tomšič, M. et al. Genetic polymorphism of CYP1A2 and the toxicity of leflunomide treatment in rheumatoid arthritis patients. Eur J Clin Pharmacol 64, 871–876 (2008). https://doi.org/10.1007/s00228-008-0498-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-008-0498-2