Abstract
In a warming climate, male sea turtles may become increasingly rare due to temperature-dependent sex determination with females being produced at warmer temperatures. Hence there is widespread concern that a lack of adult males may impact population viability. However, there is controversy over this scenario and here we review aspects of the biology of male sea turtles that will help mitigate female-biased hatchling sex ratios. In particular, there is strong evidence that males generally breed more frequently than females (i.e. have a shorter remigration interval) and that individual breeding males actively search for females and may mate with multiple females from different nesting sites. These aspects of the biology of male turtles will cause female-biased hatchling sex ratios to translate into more balanced adult sex ratios on the breeding grounds (i.e. operational sex ratios). Sexual dimorphism is widespread with adult male turtles generally being smaller than females. In freshwater turtles, this sexual dimorphism is linked to earlier age at maturity for males, although this possibility has not been examined widely in sea turtles. We make a forward-looking horizon-scanning prediction for key changes that might be expected at sea turtle breeding grounds if female-biased sex ratios become so extreme that male turtles start to become limiting and start driving populations to extinction. In particular, as the numbers of adult males on the breeding grounds become limiting there may be changes in egg fertility, multiple paternity and hatching success within clutches.
Similar content being viewed by others
Introduction
Across a broad range of taxa and ecosystems, horizon-scanning expert reviews have been used to identify key unresolved research questions including important knowledge gaps that need to be filled to help species conservation (Sutherland et al. 2012). Sometimes for otherwise well-studied taxa, there may be particular aspects of their biology that remain little understood, but which may have major conservation implications. Such is the case with sea turtles. While studies around the world have provided huge advances in our knowledge of the ecology and physiology of sea turtles, one key remaining knowledge gap concerns the biology of male turtles, which, in a warming climate, may hold the key to the survival of populations (Poloczanska et al. 2009; Hamann et al. 2010). In extreme cases, a lack of males might lead to the infertility of clutches and so population extinction.
Adult female turtles come ashore to nest allowing extensive studies on their biology, including trends in nesting numbers through beach surveys, population structure through molecular analysis, mortality rates through mark recapture, movements recorded through tracking and diving behaviour recorded with data loggers (for overview, see Rees et al. 2016). However, studying adult male turtles is often not straightforward since they generally do not come ashore and so are hard to access, for example for tissue sample collection or to attach tracking devices. Yet, despite this lack of knowledge about male turtles, there is widespread concern that male turtles may become increasingly rare as climate warming causes increasingly female-biased hatchling sex ratios due to the combination of temperature-dependent sex determination and female hatchlings being produced at warmer incubation temperatures (Poloczanska et al. 2009; Jensen et al. 2018).
Given the identified need for better information about the biology of male turtles, here we review some of the recent advances in this area, including studies on the migration of male turtles, breeding periodicity and their behaviour on the breeding grounds. We use this review to identify key methodologies that may have wide utility to extend studies on adult males around the world. Further, we make a forward-looking horizon-scanning prediction for key changes that might be expected at sea turtle breeding grounds if female-biased sex ratios become so extreme that male turtles start to become limiting and start driving populations to extinction. In this way, we provide suggestions for important long-term studies that are needed.
Phenology of breeding and remigration intervals
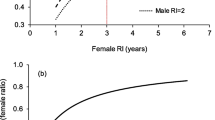
Information on the relative timing of breeding and nesting in sea turtles has come from observations made by shore-based (e.g. Limpus 1993; Godley et al. 2002), boat/snorkel (Hays et al. 2010) and drone surveys (Bevan et al. 2016; Schofield et al. 2017), as well as long-term satellite tracking (Plotkin et al. 1996; Schofield et al. 2013) (Fig. 1a). These studies point to males arriving at the breeding grounds before females, presumably in an effort to maximise their chances of encountering, and mating with, arriving females. Males then return to their foraging grounds many weeks or several months before females, who continue to lay multiple clutches across the nesting season (Fig. 1a). So in a single breeding season, male turtles (i) likely are away from their foraging sites for less time than females and (ii) likely invest less in reproduction as they are not investing energy in nesting and egg production (Hays et al. 2014). As a consequence of these two factors, after a breeding season the body condition likely recovers to pre-breeding levels far more quickly in males than females and hence the interval between breeding seasons (termed the remigration interval) is predicted to be less for males (Hays et al. 2014). So, for example, theoretical considerations suggest that where the remigration interval is 2 years for females, then it is likely 1 year for males and where it is 4 years for females, it is likely 2 years for males, i.e. males likely have a breeding season about twice as often as females (Hays et al. 2014).

Modified from Schofield et al. (2013). Similarly, elsewhere in the world and with other species, the mating season has been shown to immediately precede the nesting season, with males departing during the early-mid nesting period (Henwood 1987; Godley et al. 2002; James et al. 2005). b In a unique and very illuminating study, Limpus et al (2005) captured adult green turtles on their foraging grounds in NE Australia and determined, through laparoscopy, whether individual adults would migrate to breed that year. Across years, the proportion of males that would breed was almost always higher than females, i.e. the remigration interval was shorter for males. The annual breeding rate is the proportion of adult turtles breeding each year. So, for example, an annual breeding rate of 0.2 means that 20% of adult turtles would breed that year and so, by extension, the remigration interval was 5 years. The higher annual breeding rate of males reflects their shorter remigration intervals
Phenology of breeding and remigration intervals. a The arrival and departure times of male and female loggerhead sea turtles at a breeding area in Greece (the island of Zakynthos) that supports one of the largest nesting populations in the Mediterranean. From satellite tracking, cumulative percentages showing male arrivals (n = 11 individuals, blue circles) and departures (n = 42, turquoise circles), female first nesting (n = 37, red circles) and departures (n = 32, pink circles). Direct observations of mating pairs (solid black line, n = 94 records).
Empirical evidence supports these theoretical considerations for a shorter remigration interval in male turtles, and hence an increased likelihood than males breed in any particular year. For example, by flipper tagging green turtles (Chelonia mydas) in the southern Great Barrier Reef, Limpus (1993) reported mean and modal remigration intervals for males of 2.1 and 1 year respectively versus 4.7 and 5 years, respectively, for females. In a complimentary study, Limpus et al. (2005) assessed the breeding condition of green turtles on their forging grounds through laparoscopy and so whether they would breed or not in any particular year and found that males were far more likely to breed than females (Fig. 1b). Furthermore, the probability of breeding co-varied between males and females across years, likely as a consequence of inter-annual variation in foraging conditions. Long-term satellite tracking of loggerhead turtles (Caretta caretta) equipped with tags on their breeding grounds in Greece revealed that 0% of females but 76% of males bred in successive years (0 of 8 tracked females versus 13 of 17 tracked males) (Hays et al. 2014). Comparing resightings of flipper tagged males and female green turtles in their breeding areas in Hawaii, Balazs (1983) reported the modal remigration interval was 1 year for males and 3 years for females. Similarly, satellite tracking male hawksbill turtles in the Caribbean and recaptures of flipper tagged male green turtles at a breeding site in Brazil have both suggested some males breed every year (van Dam et al. 2008; Grossman et al. 2019).
In short, both theoretical considerations and empirical studies suggest that remigration intervals are generally shorter for males than females. Consequently, it might be expected that female-skewed hatchling sex ratios will translate into more balanced operational sex ratios (OSRs), i.e. the sex ratio on the breeding grounds.
Operational sex ratios (OSRs)
It is well known that around the world and for different species, female-biased hatchling sex ratios predominate for sea turtles (Hays et al 2014). Set against this backdrop, in recent years, a number of studies have used innovative methods to assess OSRs. In elegant work, Wright et al. (2012) used parentage analysis of samples collected from loggerhead hatchlings in northern Cyprus to estimate the number of breeding males fathering clutches of a known number of females. They estimated that while the hatchling sex ratio was 95% female, the OSR was only 42% female, in line with a shorter remigration interval for males meaning that female-skewed hatchling sex ratios translate into far more balanced OSRs (Fig. 2). Similarly, Lasala et al. (2013) used this same type of parentage analysis to estimate that for loggerhead turtles nesting at a site in Georgia, USA, the OSR was only 27% female. Using a different approach of photo-identification collected during snorkel surveys on the breeding grounds for loggerhead turtles in Greece, Hays et al. (2010) estimated an OSR of 43% female compared to a hatchling sex ratio of 70% female. This conclusion of a fairly balanced OSR at this site was later re-iterated using drone surveys to count males and females during the breeding season (Schofield et al. 2017). Similarly for green turtles breeding at Heron Island, Australia, drone and boat surveys have been used to estimate OSRs of 39 and 49% female respectively (Yaney-Keller et al. 2021) despite estimates of highly female-biased hatchling sex ratios (Booth and Astill 2001). So the picture emerging is that while female-biased hatchling sex ratios dominate in sea turtle populations across the globe, so far studies are reporting far more balanced OSRs and so males may not be as limited as previously thought (Arendt et al. 2021). It is important to note also that the hatchling sex ratio and the OSR during the same breeding season may not be exactly comparable because the sex of the adults was determined more than 30 years ago when the incubation temperature may have been lower than in the present day. Nonetheless, the success of these innovative studies outlined above provides a good roadmap for similar work to be extended to other populations around the world.
Proportion of males and females among hatchlings and breeding adults for green turtles nesting in northern Cyprus (eastern Mediterranean, located indicated in the inset map). The hatchling sex ratio was estimated from incubation durations versus the breeding sex ratio (i.e. operational sex ratio or OSR) estimated from paternity analysis of clutches (redrawn from Wright et al. 2012). Red bars females, Blue bars males. Likewise, other studies around the world and with different species have shown that female-biased hatchling sex ratios generally translate into far more balanced OSRs
Mating systems and multiple paternity
Many studies have assessed the extent of multiple paternity within sea turtle clutches, typically assigning parentage using a small blood sample from each hatchling (Fig. 3). The extent of multiple paternity varies across studies. For example, the incidence of multiple paternity in green turtle nests was 92% at Tortugeuro (Costa Rica), 61% at Ascension Island (South Atlantic) and 24% at Alagadi Beach (northern Cyprus) (Lee and Hays, 2004; Wright et al. 2013; Alfaro-Nunez et al. 2015). A review of values reported across the globe and across species, suggests that the extent of multiple paternity is linked to the packing density of adult males and females on the breeding grounds, i.e. is a consequence of both the numbers of turtles and the area they occupy during the breeding season (Lee et al. 2018). For example, large mating aggregations that are concentrated close to nesting beaches on Zakynthos in Greece, have the highest levels of multiple paternity within clutches (Zbinden et al. 2007). Another example is the low incidence (27%) of multiple paternity for leatherback turtles (Dermochelys coriacea) nesting in the US Virgin Islands, which appears linked to a relatively smaller population and the fact that female leatherbacks disperse quite widely during the breeding season (Stewart and Dutton 2014; Lee et al. 2018). This finding is consistent with the suggestion that there is little benefit of multiple paternity for female sea turtles, but rather females simply give into persistent mating attempts so that the number of times they are mated is linked to their male encounter rate (Lee and Hays 2004). Counterargument to this is a study on loggerhead turtles in Australia that found a positive correlation between multiple paternity and the proportion of developed embryos, warranting further investigations into this topic (Howe et al 2018).

Modified from Lee et al. (2018)
Sea turtle mating systems. a A pair of loggerhead turtles mating in Greece. There is strong evidence supporting the hypothesis that female sea turtles simply “give in” to unwanted mating attempts, which is termed “convenience polyandry”. So where there is a high packing density of males and females on the breeding grounds, high levels of multiple paternity occur within clutches (Lee et al. 2018). Photo credit: Kostas Papafitsoros. b and c For locations around the world, the incidence of multiple paternity across populations of different species. Generally, multiple paternity occurs widely.
Individual male turtles can mate with many females (Limpus 1993; Crim et al. 2002; Papafitsoros et al. 2022). For example, within a single breeding season, Limpus (1993) reported that male green turtles were sexually active for about a month and could mate with several females. Limpus (1993) also reported that over a 33 day period one male green turtle at Heron Island on the southern Great Barrier Reef (Australia) was observed mounted on at least six different females, with both males and females being identified by numbered flipper tags.
In the context of climate change and female-skewed hatchling sex ratios, the occurrence of multiple paternity in sea turtle clutches can be viewed as a positive finding, in that it suggests that adult male turtles are not limiting in a population. For example, for 25 of 31 studies, Lee et al. (2018) reported an incidence of multiple paternity of ≥ 20% of all clutches. The incidence of multiple paternity is likely to vary within and across seasons depending on how the relative numbers of females and males varies, possibly in relation to the timing and intensity of the influx of females (Gonzalez-Cortes et al. 2021; Schofield et al. 2017).
Movements and behaviour of male turtles on the breeding grounds
A range of methodologies are starting to highlight differences in the patterns of movement between adult male and female sea turtles. First, satellite tracking is one approach that has revealed shorter remigration intervals for males versus females (see earlier). Further, satellite tracking has suggested that, in some populations, more males tend to complete shorter post-breeding migrations. For example, Hays et al. (2014) reported that for loggerhead turtles equipped with satellite tags on their breeding adjacent to nesting beaches in Greece, 9 of 17 tracked males migrated to foraging grounds < 500 km distant, while for females this ratio was only 1 in 8, i.e. females tended to migrate further to their foraging grounds. Similarly, others have used satellite tracking to show that males sometimes stay in the vicinity of the breeding area year round or travel relatively short distances (Shaver et al. 2005; van Dam et al. 2008; Arendt et al. 2012; Cuevas et al. 2020). In some cases, males make long migrations similar to those of females, although this generally occurs in a smaller proportion of males compared to females (van Dam et al. 2008; Arendt et al. 2012; Casale et al. 2013).
Satellite tracking and molecular analysis have also both suggested that males likely travel more widely in the breeding season than females, visiting several breeding areas in one general location. For example, Wright et al. (2012) satellite tracked a male loggerhead turtle that visited multiple nesting beaches in the eastern Mediterranean, while both FitzSimmons et al. (1997) and Lee et al. (2007) used molecular analysis to infer that males moved further in the breeding season than females. As a consequence of these differences in movements, males likely father offspring widely across the genetic stock range while females tend to mate in more confined areas.
While mating is often thought to be concentrated close to nesting beaches (e.g. Godley et al 2002), mating may sometimes also occur distant to nesting beaches. For example, for green turtles nesting in some parts of the Great Barrier Reef, mating is thought to occur at regional courtship areas before females travel onto their nesting beaches and in these cases, males may be breeding with females from many rookeries within the region (Limpus 1993). Furthermore, direct observations have shown mating out at sea 10 s of km from nesting beaches (Lee et al. 2007). Presumably, in these cases males are taking the opportunity to mate after chance encounters with females while both are en route to nesting areas and, in these cases, again different mated females may be travelling to different nesting beaches. Taken together these studies suggest that a single male will likely be able to find and mate with many females, and with females that nest on different beaches, which may again help mitigate female biased OSRs. For example, even if no males are produced from a single nesting beach, females from that beach may be mated with males produced from other beaches in the locality. This conclusion helps explain observations of certain beaches likely only producing female hatchlings across many decades when mixed hatchling sex ratios are produced from nearby beaches (Hays et al. 2003). However, the maximum number of females a male can mate with successfully is still not well known and is an important question to address in future studies.
More extensive movement of males on the breeding grounds has also been revealed at local levels using drone surveys. For loggerhead turtles in Greece, drone surveys revealed that on their breeding grounds male loggerhead turtles tend to be actively travelling while females tend to be stationary, resting on the seabed (Dickson et al. 2022). This difference in behaviour may reflect the advantage to males of finding many females, in terms of increased mating opportunities, while for females there may be little advantage to mating with many males (Lee and Hays 2004). Indeed females have been observed trying to avoid approaching males (Reina et al. 2005). Compared to males, females seem to adopt a strategy of energy conservation in the breeding season to maximise reproductive output (Jessop et al 2004), a strategy that is likely aided by the ability of females to store sperm to fertilize many clutches (Pearse and Avise, 2001; Pearse et al. 2002).
Growth rates and age at maturity
Many studies have reported the size of breeding male and female sea turtles and in some species there is a consistent finding that adult male turtles are smaller than females (Godley et al. 2002). For example, Godley reported that for green turtles around the world, the ratio of female to male carapace length averaged 1.07, i.e. females were 7% longer. However, in sea turtles the relative age at which males and females reach sexual maturity is largely unknown. In theory, where there is sexual dimorphism with adult females being larger than adult males, then if growth rates are similar between sexes then we might expect males to reach maturity earlier. Certainly, this scenario appears to be the case in freshwater turtles where it has been widely known for many years that males of some species mature earlier than females. For example, for the freshwater turtle Malaclemys terrapin, Lovich and Gibbons (1990) reported that males mature earlier and at a smaller body size than females, with males typically reaching maturity at age three and females at age six. Indeed Lovich and Gibbons (1990) proposed that male:female differences in age to reach maturity may be the key driver of adult sex ratios.
Some evidence suggests that male versus female growth rates of immature sea turtles are similar. For example, Avens et al. (2021) reported no difference in growth rates between males and females for immature hawksbill turtles in the western Atlantic, consistent with reports from the Caribbean region (Krueger et al. 2011; Hart et al. 2013) and a site in the northern Great Barrier Reef, Australia (Bell and Pike 2012). However, an older study in the southern Great Barrier Reef suggested faster growth rates for juvenile female hawksbills (Chaloupka and Limpus 1997). No sex-specific differences in growth rates were reported for juvenile loggerhead sea turtles in the western North Atlantic (Avens et al. 2013), but sub-adult males grew faster than females (Avens et al. 2015). Arendt et al. (2021) concluded that during the oceanic growth phase, male loggerhead turtles tended to grow faster than females. For immature green turtles, no sex-specific differences in growth rates were reported in the northeast Gulf of Mexico (Avens et al. 2012), Bahamas (Bolten et al. 1992) or Australia (Limpus and Chaloupka 1997). Sexual dimorphism is not universal across sea turtle species. For example, for Kemp’s ridley turtles, Avens et al. (2017) found no differences between male and females in either their size or age at sexual maturity, while Casale et al. (2005) and Ishihara and Kamezaki (2011) suggested that male and female loggerheads reach maturity at a similar size in the Mediterranean and the North Pacific respectively. Taken together, these studies largely suggest comparable growth rates of immature male and female sea turtles and hence support the suggestion (Lovich and Gibbons 1990) that sexual size dimorphism likely reflects differences in age at maturation. Clearly, this is an area warranting much more work considering that an earlier age-at-first breeding for male sea turtles would be another mechanism by which female-biased hatchling sex ratios translate into more-balanced OSRs, provided it is not offset by a shorter life expectancy in males.
Relative mortality rate
There is a general paucity of information on the relative mortality rates of males and females. For loggerhead turtles breeding in Greece, lower annual survival rates in males have been linked to their post-breeding movements with males occupying more coastal areas, which is thought to increase their risk of mortality from human sources such as boat strikes and fishery bycatch (Schofield et al. 2020). This interaction between movements and survival estimates is likely to be heavily dependent on the relative threats that males and females face in different foraging areas. So while coastal occupation has been seen in males of other species (e.g. James et al. 2005; Shaver et al. 2005; van Dam et al. 2008; Cuevas et al. 2020), the impacts on survival rates in these cases were unknown. When comparing across populations, i.e. where mortality rates for males and females have come from different sites, there is no clear difference in the mortality rates of males versus females (Schofield et al. 2020). Based on recapture rates of flipper tagged individuals, Chaloupka & Limpus (2005) reported no sex differences in survival rates for green turtles in the southern Great Barrier Reef. These few estimates of the relative survival rates of males versus females, highlight that this topic is an important knowledge gap. Furthermore, a difference in male versus female survival from hatchling to adult might also contribute to a change in the sex ratio from hatchlings to adults, although there is a dearth of information on this topic (Delgado et al. 2010).
Assessing when males may become limiting
It remains equivocal how the tipping-point can be determined when males become so limiting that population viability is compromised due to the resulting low fertility within clutches. The fact that hatchling sex ratios may be highly female-skewed in some very large nesting populations (Hays et al. 2017), suggests that even small numbers of males may be enough for population viability (Fig. 4). For example, for loggerhead turtles nesting in Florida it has been estimated that hatchling sex ratio skews are > 94% female on some nesting beaches and yet nesting populations are very large and are not in decline (Hays et al. 2017). Aspects of the life history of males, that we have reviewed here, may explain why populations remain viable even when hatchlings sex ratios are massively female skewed. In particular, males breeding more frequently than females and individual males mating with many females, will both help to mitigate such female-biased hatchling sex ratio skews. It remains uncertain how many females a single male turtle can mate with successfully in wild populations and this ratio is likely dependent on the packing density of males and females on the breeding grounds and hence their encounter rates plus also the physiological constraint of male sperm production. For example, if hatchling sex ratios are 98% female biased (i.e. 1 male hatchling for every 50 female hatchlings), then if the remigration interval for adult males is half that of females, then each breeding male would need to mate with 25 females if all females are to produce viable clutches. Using these sorts of calculations, it is clear that at extreme hatchling sex ratio skews (> 99% female), then the number of females each male needs to mate with can become very high (> 50). In such extreme cases, the potential for inbreeding and loss of genetic variation is unknown and is an important question to address. Others too have noted that climate warming and increasingly female-skewed hatchling sex ratios will require male turtles to mate with an increased number of females to maintain population viability (Jensen et al. 2022).

Modified from Hays et al. (2017)
When does a lack of adult males impact population viability ? In most studies around the world there is a marked female bias in the hatchling sex ratio. Yet the % of female hatchlings is not linked to population size, i.e. very large nesting populations may still have a marked female hatchling sex ratio bias. Hatchling sex ratios from histological techniques (open circles) or incubation/temperature-derived (black circles).
Once OSRs are too skewed for all breeding females to be mated, a point will be reached when clutch viability is compromised. In short, if females are not mated, then they will produce infertile eggs. So declining egg fertility across years may be a sign that males are becoming more limited. Interestingly, even at Raine Island, Australia, the largest green turtle rookery in the world and a site where female-biased hatchling sex ratios are thought to be extreme (99%, Jensen et al. 2018), egg fertility in clutches appears to remain high (Booth et al. 2021). In cases where egg fertility is not measured, we might expect that the incidence of multiple paternity would provide an alternative indication that males are becoming limited, for example if there is a change within nesting populations from a higher to a lower incidence of multiple paternity over time. Where neither egg fertility nor the incidence of multiple paternity are measured, hatching success provides a simple measure that could be used to indicate whether males are becoming limited, since infertile eggs will not hatch. Hatching success is estimated after hatchlings have emerged, i.e. the proportion of eggs laid that result in a hatchling emerging. While low hatching success can have many causes (e.g. nest inundation, disease, high incubation temperatures), a decline in hatching success throughout a given rookery (i.e. all possible environmental conditions) across years might also indicate a decline in egg fertility. Hence the long-term monitoring of egg fertility, incidence of multiple paternity and hatching success all have value for ongoing studies.
Summary and conclusions
Aspects of the biology of male turtles may become increasingly important in mitigating increasingly female-biased hatchling sex ratios that are predicted to accompany climate warming. A number of factors will drive how hatchling sex ratios translate into operational sex ratios and only some of these factors have been assessed in sea turtles (Fig. 5). There is good data on sexual dimorphism in some species of sea turtles, with males often being smaller. Immature growth rates are generally similar between males and females suggesting that males will tend to reach maturity sooner although relative ages at maturity remains an important knowledge gap. Limited data on relative mortality rates have shown higher male mortality rates linked to their movements. Strong evidence suggests males have a shorter remigration interval than females, which will help mitigate heavily female-biased hatchling sex ratios. Future studies could target, with a high priority, important knowledge gaps including sexual differences in (1) relative age at maturity, (2) relative mortality rates and (3) further information on relative remigration intervals. It should also be noted that the biology of males is generally best described for green and loggerhead turtles (e.g. Limpus 1993; Hays et al. 2010). Although theoretical considerations suggest that key aspects of the biology of males, such as their shorter remigration intervals compared to females, are likely to occur across species (Hays et al. 2014), further studies on the biology of males of other species are needed. Ongoing, long-term monitoring of egg fertility within clutches, the incidence of multiple paternity, and hatching success may provide an indication that a lack of breeding males is impacting population viability. A concern that remains, aside from hatchling sex ratio skews, is that increases in incubation temperature, occurring as part of climate warming, will raise embryonic death in eggs (Hays et al. 2017) and so negatively impact populations.
Likely drivers of egg fertility in clutches. Schematic representation of some of the factors that likely drive how hatchling sex ratios translate into operational sex ratios and thereby subsequent egg fertility. Red circles depict females and blue circle males and illustrate a female-biased hatchling sex ratio leading to a more balanced operational sex ratio, as has been found in many locations. For example, as illustrated earlier age at maturity (shown in some freshwater turtles but a knowledge gap at present for sea turtles) and shorter remigration intervals (now fairly well established) for males will mean that female-biased hatchling sex ratios translate into more balanced OSRs. These affects will be countered if mortality rates are higher in males, although relative mortality rates is another important knowledge gap. Key aspects of mating patterns that likely drive clutch fertility include the movements of males and females, which will influence encounter rates, and how many females can be mated successfully by individual males
Data availability
The data are provided in the original papers cited in this review.
References
Alfaro-Nunez A, Jensen MP, Abreu-Grobois FA (2015) Does polyandry really pay off? The effects of multiple mating and number of fathers on morphological traits and survival in clutches of nesting green turtles at Tortuguero. PeerJ 3:e880. https://doi.org/10.7717/peerj.880
Arendt MD, Segars AL, Byrd JI, Boynton J, Schwenter JA, Whitaker JD, Parker L (2012) Migration, distribution, and diving behavior of adult male loggerhead sea turtles (Caretta caretta) following dispersal from a major breeding aggregation in the Western North Atlantic. Mar Biol 159:113–125. https://doi.org/10.1007/s00227-011-1826-0
Arendt MD, Schwenter JA, Owens DW, Valverde R (2021) Theoretical modeling and neritic monitoring of loggerhead Caretta caretta [Linnaeus, 1758] sea turtle sex ratio in the southeast United States do not substantiate fears of a male-limited population. Glob Change Biol 27:4849–4859. https://doi.org/10.1111/gcb.15808
Avens L, Goshe LR, Harms CA, Anderson ET, Hall AG, Cluse WM, Godfrey MH, Braun-McNeill J, Stacy B, Bailey R, Lamont MM (2012) Population characteristics, age structure, and growth dynamics of neritic juvenile green turtles in the northeastern Gulf of Mexico. Mar Ecol Prog Ser 458:213–229. https://doi.org/10.3354/meps09720
Avens L, Goshe LR, Pajuelo M, Bjorndal KA et al (2013) Complementary skeletochronology and stable isotope analyses offer new insight into juvenile loggerhead sea turtle oceanic stage duration and growth dynamics. Mar Ecol Prog Ser 491:235–251. https://doi.org/10.3354/meps10454
Avens L, Goshe LR, Coggins L, Snover ML, Pajuelo M, Bjorndal KA, Bolten AB (2015) Age and size at maturation and adult-stage duration for loggerhead sea turtles in the western North Atlantic. Mar Biol 162:1749–1767. https://doi.org/10.1007/s00227-015-2705-x
Avens L, Goshe LR, Coggins L, Shaver DJ, Higgins B, Landry AM Jr, Bailey R (2017) Variability in age and size at maturation, reproductive longevity, and long-term growth dynamics for Kemp’s ridley sea turtles in the Gulf of Mexico. PLoS ONE 12:e0173999. https://doi.org/10.1371/journal.pone.0173999
Avens L, Ramirez MD, Goshe LR, Clark JM, Meylan AB, Teas W, Shaver DJ, Godfrey MH, Howell L (2021) Hawksbill sea turtle life-stage durations, somatic growth patterns, and age at maturation. Endang Species Res 45:127–145. https://doi.org/10.3354/esr01123
Balazs GH (1983) Recovery records of adult green turtles observed originally tagged at French Frigate Shoals, Northwestern Hawaiian Islands. NOAA-TM-NMFS-SWFC-36, 47p
Bell I, Pike DA (2012) Somatic growth rates of hawksbill turtles Eretmochelys imbricata in a northern Great Barrier Reef foraging area. Mar Ecol Prog Ser 446:275–283. https://doi.org/10.3354/meps09481
Bevan E, Wibbels T, Navarro E, Rosas M, Najera BMZ, Sarti L, Illescas F, Montano J, Pena LJ, Burchfield P (2016) Using unmanned aerial vehicle (UAV) technology for locating, identifying, and monitoring courtship and mating behaviour in the green turtle (Chelonia mydas). Herpetological Review 47:27–32
Bolten AB, Bjorndal KA, Grumbles JS, Owens DW (1992) Sex ratio and sex-specific growth rates in immature green turtles, Chelonia mydas, in the southern Bahamas. Copeia 1992:1098–1103
Booth DT, Astill K (2001) Temperature variation within and between nests of the green sea turtle, Chelonia mydas (Chelonia: Cheloniidae) on Heron Island, Great Barrier Reef. Aust J Zool 49:71–84. https://doi.org/10.1071/ZO00059
Booth DT, Dunstan A, Robertson K, Tedeschi J (2021) Egg viability of green turtles nesting on Raine Island, the world’s largest nesting aggregation of green turtles. Aust J Zool 69:12–17. https://doi.org/10.1071/ZO21024
Casale P, Freggi D, Basso R, Argano R (2005) Size at male maturity, sexing methods and adult sex ratio in loggerhead turtles (Caretta caretta) from Italian waters investigated through tail measurements. Herpetol J 15:145–148
Casale P, Freggi D, Cina A, Rocco M (2013) Spatio-temporal distribution and migration of adult male loggerhead sea turtles (Caretta caretta) in the Mediterranean Sea: further evidence of the importance of neritic habitats off North Africa. Mar Biol 160:703–718. https://doi.org/10.1007/s00227-012-2125-0
Chaloupka M, Limpus CJ (1997) Robust statistical modelling of hawksbill sea-turtle growth rates (southern Great Barrier Reef). Mar Ecol Prog Ser 146:1–8
Chaloupka M, Limpus C (2005) Estimates of sex- and age-class-specific survival probabilities for a southern Great Barrier Reef green sea turtle population. Mar Biol 146:1251–1261. https://doi.org/10.1007/s00227-004-1512-6
Crim JL, Spotila LD, Spotila JR, O’Connor M, Reina R, Williams CJ, Paladino FV (2002) The leatherback turtle, Dermochelys coriacea, exhibits both polyandry and polygyny. Mol Ecol 11:2097–2106
Cuevas E, Putman NF, Uribe-Martínez A, López-Castro MC, Guzmán-Hernández V, Gallegos-Fernández SA, Liceaga-Correa MÁ, Trujillo-Córdova JA, González-Díaz-Mirón RJ, Negrete-Phillipe A, Acosta-Sánchez HH, Martínez-Portugal RC, López-Hernández M, Huerta-Rodríguez P, Silver J (2020) First spatial distribution analysis of male sea turtles in the southern Gulf of Mexico. Front Mar Sci 7:561846. https://doi.org/10.3389/fmars.2020.561846
Delgado C, Canário AVM, Dellinger T (2010) Sex ratios of loggerhead sea turtles Caretta caretta during the juvenile pelagic stage. Mar Biol 157:979–990. https://doi.org/10.1007/s00227-009-1378-8
Dickson LC, Tugwell H, Katselidis KA, Schofield G (2022) Aerial drones reveal the dynamic structuring of sea turtle breeding aggregations and minimum survey effort required to capture climatic and sex-specific effects. Front Mar Sci 9:864694. https://doi.org/10.3389/fmars.2022.864694
FitzSimmons NN, Moritz C, Limpus CJ, Pope L, Prince R (1997) Geographic structure of mitochondrial and nuclear gene polymorphisms in Australian green turtle populations and male-biased gene flow. Genetics 147:1843–1854
Godley BJ, Broderick AC, Frauenstein R, Glen F, Hays GC (2002) Reproductive seasonality and sexual dimorphism in green turtles. Mar Ecol Prog Ser 226:125–133. https://doi.org/10.3354/meps226125
Gonzalez-Cortes L, Labastida-Estrada E, Karam-Martinez SG, Montoya-Marquez JA, Islas-Villanueva V (2021) Within-season shifts in multiple paternity patterns in mass-nesting olive ridley sea turtles. Endang Species Res 46:79–90. https://doi.org/10.3354/esr01144
Grossman A, Daura-Jorge F, de Brito SM, Ortigara Longa G (2019) Population parameters of green turtle adult males in the mixed ground of Atol das Rocas, Brazil. Mar Ecol Prog Ser 609:197–207. https://doi.org/10.3354/meps12821
Hamann M, Godfrey MH, Seminoff JA, Arthur K, Barata PCR, Bjorndal KA, Bolten AB, Broderick AC, Campbell LM, Carreras C, Casale P, Chaloupka M, Chan SKF, Coyne MS, Crowder LB, Diez CE, Dutton PH, Epperly SP, FitzSimmons NN, Formia A, Girondot M, Hays GC, Cheng IJ, Kaska Y, Lewison R, Mortimer JA, Nichols WJ, Reina RD, Shanker K, Spotila JR, Wallace TJ, BP, Work TM, Zbinden J, Godley BJ, (2010) Global research priorities for sea turtles: informing management and conservation in the 21st century. Endanger Species Res 11:245–269. https://doi.org/10.3354/esr00279
Hart KM, Sartain AR, Hillis-Starr ZM, Phillips B et al (2013) Ecology of juvenile hawksbills (Eretmochelys imbricata) at Buck Island Reef national monument, US Virgin Islands. Mar Biol 160:2567–2580. https://doi.org/10.1007/s00227-013-2249-x
Hays GC, Broderick AC, Glen F, Godley BJ (2003) Climate change and sea turtles: a 150 year reconstruction of incubation temperatures at a major marine turtle rookery. Glob Change Biol 9:642–646. https://doi.org/10.1046/j.1365-2486.2003.00606.x
Hays GC, Fossette S, Katselidis KA, Schofield G, Gravenor MB (2010) Breeding periodicity for male sea turtles, operational sex ratios, and implications in the face of climate change. Conserv Biol 24:1636–1643. https://doi.org/10.1111/j.1523-1739.2010.01531.x
Hays GC, Mazaris AD, Schofield G, Laloë J-O (2017) Population viability at extreme sex-ratio skews produced by temperature-dependent sex determination. Proc Roy Soc B 284:20162576. https://doi.org/10.1098/rspb.2016.2576
Hays GC, Mazaris AD, Schofield G (2014) Different male vs female breeding periodicity helps mitigate offspring sex ratio skews in sea turtles. Front Mar Sci. https://doi.org/10.3389/fmars.2014.00043
Howe M, FitzSimmons NN, Limpus CJ, Clegg SM (2018) Multiple paternity in a Pacific marine turtle population: maternal attributes, offspring outcomes and demographic inferences. Mar Biol 165:2. https://doi.org/10.1007/s00227-017-3258-y
Ishihara T, Kamezaki N (2011) Size at maturity and tail elongation of loggerhead turtles (Caretta caretta) in the North Pacific. Chelonian Conservation and Biology 10:281–287. https://doi.org/10.2744/ccb-0893.1
James MC, Eckert SA, Myers RA (2005) Migratory and reproductive movements of male leatherback turtles (Dermochelys coriacea). Mar Biol 147:845–853
Jensen MP, Allen CD, Eguchi T, Bell IP, LaCasella EL, Hilton WA, Hof CAM, Dutton PH (2018) Environmental warming and feminization of one of the largest sea turtle populations in the world. Curr Biol 28:154–159. https://doi.org/10.1016/j.cub.2017.11.057
Jensen MP, Eguchi T, FitzSimmons N, McCarthy M, Fuentes M, Hamann M, Limpus CJ, Bell I, Read M (2022) Integrating climate change and management scenarios in population models to guide the conservation of marine turtles. Bull Mar Sci. https://doi.org/10.5343/bms.2021.0033
Jessop TS, Hamann M, Limpus CJ (2004) Body condition and physiological changes in male green turtles during breeding. Mar Ecol Prog Ser 276:281–288
Krueger BH, Chaloupka MY, Leighton PA, Dunn JA, Horrocks JA (2011) Somatic growth rates for a hawksbill turtle population in coral reef habitat around Barbados. Mar Ecol Prog Ser 432:269–276. https://doi.org/10.3354/meps09125
Lasala JA, Scott Harrison J, Williams KL, Rostal DC (2013) Strong male-biased operational sex ratio in a breeding population of loggerhead turtles (Caretta caretta) inferred by paternal genotype reconstruction analysis. Ecol Evol 3:4736–4747. https://doi.org/10.1002/ece3.761
Lee PLM, Hays GC (2004) Polyandry in a marine turtle: females make the best of a bad job. Proc Natl Acad Sci USA 101:6530–6535. https://doi.org/10.1073/pnas.0307982101
Lee PLM, Luschi P, Hays GC (2007) Detecting female precise natal philopatry in green turtles using assignment methods. Mol Ecol 16:61–74. https://doi.org/10.1111/j.1365-294X.2006.03115.x
Lee PLM, Schofield G, Haughey RI, Mazaris AD, Hays GC (2018) A review of patterns of multiple paternity across sea turtle rookeries. Adv Mar Biol 79:1–31. https://doi.org/10.1016/bs.amb.2017.09.004
Limpus CJ (1993) The green turtle, Chelonia mydas, in Queensland: breeding males in the southern Great Barrier Reef. Wildl Res 20:513–523
Limpus C, Chaloupka M (1997) Nonparametric regression modelling of green sea turtle growth rates (southern Great Barrier Reef). Mar Ecol Prog Ser 149:23–34
Limpus CJ, Limpus DJ, Arthur KE, Parmenter CJ (2005) Monitoring green turtle population dynamics in Shoalwater Bay 2000–2004. Research Publication 83, Great Barrier Reef Marine Park Authority, Townsville, QLD.
Lovich JE, Gibbons JW (1990) Age at maturity influences adult sex ratio in the turtle Malaclemys terrapin. Oikos 59:126–134
Papafitsoros K, Dimitriadis C, Mazaris AD, Schofield G (2022) Photo-identification confirms polyandry in loggerhead sea turtles. Mar Ecol. https://doi.org/10.1111/maec.12696
Pearse DE, Avise JC (2001) Turtle mating systems: behavior, sperm storage, and genetic paternity. J Hered 92:206–211
Pearse DE, Janzen FJ, Avise JC (2002) Multiple paternity, sperm storage, and reproductive success of female and male painted turtles (Chrysemys picta) in nature. Behav Ecol Sociobiol 51:164–171. https://doi.org/10.1007/s00265-001-0421-7
Plotkin PT, Owens DWM, Byles RA, Patterson R (1996) Departure of male olive ridley turtles (Lepidochelys olivacea) from a nearshore breeding ground. Herpetologica 52:1–7
Poloczanska ES, Limpus CJ, Hays GC (2009) Vulnerability of marine turtles to climate change. Adv Mar Biol 56:151–211. https://doi.org/10.1016/S0065-2881(09)56002-6
Rees AF, Alfaro-Shigueto J, Barata PCR, Bjorndal KA, Bolten AB, Bourjea J, Broderick AC, Campbell LM, Cardona L, Carreras C, Casale P, Ceriani SA, Dutton PH, Eguchi T, Formia A, Fuentes MMPB, Fuller WJ, Girondot M, Godfrey MH, Hamann M, Hart KM, Hays GC, Hochscheid S, Kaska Y, Jensen MP, Mangel JC, Mortimer JA, Naro-Maciel E, Ng CKY, Nichols WJ, Phillott AD, Reina RD, Revuelta O, Schofield G, Seminoff JA, Shanker K, Tomás J, van de Merwe JP, Van Houtan KS, Vander Zanden HB, Wallace BP, Wedemeyer-Strombel KR, Work TM, Godley BJ (2016) Are we working towards global research priorities for management and conservation of sea turtles ? Endang Species Res 31:337–382. https://doi.org/10.3354/esr00801
Reina RD, Abernathy KJ, Marshall GJ, Spotila JR (2005) Respiratory frequency, dive behaviour and social interactions of leatherback turtles, Dermochelys coriacea during the inter-nesting interval. J Exp Mar Biol Ecol 316:1–16
Schofield G, Scott R, Dimadi A, Fossette S, Katselidis KA, Koutsoubas D, Lilley MKS, Pantis JD, Karagouni AD, Hays GC (2013) Evidence-based marine protected area planning for a highly mobile endangered marine vertebrate. Biol Conserv 161:101–109. https://doi.org/10.1016/j.biocon.2013.03.004
Schofield G, Katselidis KA, Lilley MKS, Reina RD, Hays GC (2017) Detecting elusive aspects of wildlife ecology using drones: new insights on the mating dynamics and operational sex ratios of sea turtles. Funct Ecol 31:2310–2319. https://doi.org/10.1111/1365-2435.12930
Schofield G, Klaassen M, Papafitsoros K, Lilley MKS, Katselidis KA, Hays GC (2020) Long-term photo-id and satellite tracking reveal sex-biased survival linked to movements in an endangered species. Ecology 101:e03027. https://doi.org/10.1002/ecy.3027
Shaver DJ, Schroeder BA, Byles RA, BurchWeld PM, Pena J, Marquez R, Martinez HJ (2005) Movements and home ranges of adult male Kemp’s ridley sea turtles (Lepidochelys kempii) in the Gulf of Mexico investigated by satellite telemetry. Chelonian Conserv Biol 4:817–827
Stewart KR, Dutton PH (2014) Breeding sex ratios in adult leatherback turtles (Dermochelys coriacea) may compensate for female-biased hatchling sex ratios. PLoS ONE 9:e88138. https://doi.org/10.1371/journal.pone.0088138
Sutherland WJ, Aveling R, Bennun L, Chapman E, Clout M, Cote IM et al (2012) A horizon scan of global conservation issues for 2012. Trends Ecol Evol 27:12–18. https://doi.org/10.1016/j.tree.2011.10.011
van Dam RP, Diez CE, Balazs GH, Colón-Colón LA, McMillan WO, Schroeder B (2008) Sex-specifific migration patterns of hawksbill turtles breeding at Mona Island, Puerto Rico. Endanger Species Res 4:85–94. https://doi.org/10.3354/esr00044
Wright LI, Stokes KL, Fuller WJ, Godley BJ, McGowan A, Snape R, Tregenza T, Broderick AC (2012) Turtle mating patterns buffer against disruptive effects of climate change. Proc Biol Sci 279:2122–2127. https://doi.org/10.1098/rspb.2011.2285
Wright LI, Fuller WJ, Godley BJ, Mcgowan A, Tregenza T, Broderick AC (2013) No benefits of polyandry to female green turtles. Behav Ecol 24:1022–1029. https://doi.org/10.1093/beheco/art003
Yaney-Keller A, San Martin R, Reina RD (2021) Comparison of UAV and boat surveys for detecting changes in breeding population dynamics of sea turtles. Remote Sensing 13:2857. https://doi.org/10.3390/rs13152857
Zbinden JA, Largiadèr CR, Leippert F, Margaritoulis D, Arlettaz R (2007) High frequency of multiple paternity in the largest rookery of Mediterranean loggerhead sea turtles. Mol Ecol 16:3703–3711. https://doi.org/10.1111/j.1365-294X.2007.03426.x
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. GCH was supported by the Bertarelli Foundation as part of the Bertarelli Programme in Marine Science (BPMS-2017–4).
Author information
Authors and Affiliations
Contributions
GCH conceived the manuscript and led the writing with contributions from GS and TS.
Corresponding author
Ethics declarations
Competing interests
No conflicts of interest or competing interests to declare.
Ethical approval
The work only used data extracted from the literature.
Additional information
Responsible Editor: S. Shumway.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hays, G.C., Shimada, T. & Schofield, G. A review of how the biology of male sea turtles may help mitigate female-biased hatchling sex ratio skews in a warming climate. Mar Biol 169, 89 (2022). https://doi.org/10.1007/s00227-022-04074-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-022-04074-3