Abstract
Bycatch is one of the key threats to juvenile marine turtles in the Mediterranean Sea. As fishing methods are regional or habitat specific, the susceptibility of marine turtles may differ according to inter- and intra-population variations in foraging ecology. An understanding of these variations is necessary to assess bycatch susceptibility and to implement region-specific management. To determine if foraging ecology differs with region, sex, and size of juvenile loggerhead turtles (Caretta caretta), stable isotope analysis of carbon and nitrogen was performed on 171 juveniles from a range of foraging regions across the central and eastern Mediterranean Sea. Isotope ratios differed with geographical region, likely due to baseline variations in δ13C and δ15N values. The absence of sex-specific differences suggests that within an area, all comparably sized animals likely exploit similar foraging strategies, and therefore, their susceptibility to fisheries threats will likely be similar. The isotope ratios of juveniles occupying the North East Adriatic and North Levantine basin increased with size, potentially due to increased consumption of more prey items at higher trophic levels from a more neritic source. Isotope ratios of juveniles with access to both neritic and oceanic habitats did not differ with size which is consistent with them consuming prey items from both habitats interchangeably. With foraging habitats exploited differently among size classes in a population, the susceptibility to fisheries interactions will likely differ with size; therefore, region-specific management approaches will be needed.
Similar content being viewed by others
Introduction
For globally distributed species, variation in life-history and behavioural traits can improve resilience and survival in a changing environment (Jiguet et al. 2007; Bernhardt and Leslie 2013; Timpane-Padgham et al. 2017). Variability in the spatial and foraging ecology of a species may occur based on many factors, including morphological (e.g. size) or demographic (e.g. sex) parameters, or as a response to the environment, and can help to reduce intraspecific competition (Werner and Gilliam 1984; Violle et al. 2012). For example, idividuals may consume different prey items resulting in individual specialisation in a generalist population (e.g. Vander Zanden et al. 2010; Thomson et al. 2018). As different individuals may play different roles within an ecosystem (Chapin et al. 2001; Violle et al. 2012), their susceptibility to disturbances, whether natural or anthropogenic, will also differ. Therefore, these variations in resource exploitation could influence population growth and dynamics (Araújo et al. 2011), complicating conservation management and requiring region-specific management approaches.
Loggerhead turtles (Caretta caretta) demonstate complex life-history patterns, utilising a wide range of ecosystems throughout their life cycle and facing various natural and anthropogenic threats at each life stage (Bolten 2003). Loggerhead turtle life-history patterns and foraging strategies vary globally, and large gaps remain in our knowledge owing to the difficulty of monitoring such long-lived animals at sea (Wildermann et al. 2018). Globally, fisheries bycatch is one of the most significant threats faced by marine turtles (Lewison et al. 2014). The extent of fishing and the fishing techniques used, drastically differs with location and habitat type (Casale 2011). Therefore, to better understand fisheries interactions and for successful conservation of loggerhead turtle populations, it is neccesary to understand inter- and intra-population variations in habitats used and resources exploited (Hamann et al. 2010; Rees et al. 2016).
To investigate the spatial and foraging ecology of juvenile loggerhead turtles, satellite telemetry deployed at foraging grounds has previously been used and can provide fine-scale near real-time movement data (e.g. McClellan and Read 2007; Mansfield et al. 2009; Arendt et al. 2012). However, satellite telemetry does not provide dietary information and the expense of this tool can often limit the sample size (Godley et al. 2008). Detailed information can be gained about the foraging ecology of individuals by analysing stomach contents and stable isotopes from stranded or incidentally captured individuals (Tomás et al. 2001; Revelles et al. 2007; Seney and Musick 2007; Casale et al. 2008; Lazar et al. 2011; Cardona et al. 2012, 2015; Blasi et al. 2018). Investigating stomach contents enables taxonomic identification of prey items but does bias against rapidly digested soft-bodied prey, represents a short dietary time frame (Duffy and Jackson 1986), and requires expertise, time, and access to freshly dead individuals. Stable isotope analysis (SIA) is a powerful cost-effective forensic tool that has been used to gain insights into the spatial and foraging ecology of numerous marine taxa (Rubenstein and Hobson 2004; Newsome et al. 2010; Bird et al. 2018), including marine turtles (Figgener et al. 2019a,b; Haywood et al. 2019). The ratio of stable isotopes within low-metabolically active tissues (e.g. epidermis and keratinised tissues such as scutes) reflects the food that an individual has consumed and the location where it was ingested (DeNiro and Epstein 1978). These tissues typically have slow turnover rates and the isotope incorporation from dietary items takes several months, and, therefore, represents diet over longer time frames than stomach content analysis (Reich et al. 2008).
The carbon isotope ratio (expressed as δ13C) of a consumer reflects the primary producer at the base of their food chain (DeNiro and Epstein 1978), with benthic and near-shore food chains supported by macroalgae and seagrass exhibiting high δ13C values in comparison to pelagic and oceanic food chains supported by phytoplankton (DeNiro and Epstein 1978; Graham et al. 2010). The nitrogen isotope ratio (expressed as δ15N) at the base of a food chain differs in relation to (1) δ15N values of their nutrient sources (e.g., N2, ammonium, and nitrate), (2) nitrogen-based processes, including; nitrification, denitrification, and N2 fixation, and (3) isotopic fractionation (Montoya 2007). On local-scales, nitrogen isotope ratios, and to a lesser extent, carbon isotope ratios, can reflect trophic patterns within a food chain due to isotopic fractionation. With each subsequent trophic level, a 3–4‰ and a ~ 1‰ step wise increase in δ15N and δ13C values, respectively, are considered to occur (DeNiro and Epstein 1978; Minagawa and Wada 1984; France and Peters 1997).
As local-scale variations in stable isotope ratios can be inferred as differences in foraging grounds used or prey items consumed, they allow for the spatial and foraging ecology of loggerhead turtles to be assessed (e.g. Thomson et al. 2012; Ramirez et al. 2015; Turner Tomaszewicz et al. 2017). This is particularly useful for juvenile loggerhead turtles in the Mediterranean Sea, which have complex spatial and foraging ecology (see Casale et al. 2018 for a review of the biology of loggerhead turtles in the Mediterranean). Juveniles can be found throughout the Mediterranean in oceanic or neritic foraging grounds (Casale et al. 2018). Identifying foraging grounds is challenging and large data gaps remain in many areas of the Mediterranean, in particular the oceanic waters of the Levantine Basin (Casale et al. 2018). Fisheries bycatch data suggest major oceanic foraging grounds include the northern Ionian/South Adriatic, the southern Ionian/Sicilian Strait, and the westernmost part of the Mediterranean (Casale et al. 2011) and satellite telemetry highlighted the Tyrrhenian Sea, Algerian Sea, the Ionian as areas of importance (Zbinden et al. 2008; Hays et al. 2014a; Mingozzi et al. 2016; Luschi et al. 2018). Foraging in these oceanic regions is likely driven by the occurrence of patchy ephemeral resources due to eddies concentrating resources (Eckert et al. 2008). Neritic foraging grounds were located in areas of high productivity and on the continental shelves of the Aegean Sea, Adriatic Sea, eastern Levantine basin, northern Africa, and off Tunisia (see Casale et al. (2018) and citations within).
Juvenile Mediterranean loggerhead turtles are considered highly opportunistic foragers with diverse dietary items reported across the Mediterranean (e.g., Tomás et al. 2001; Casale et al. 2008; Lazar et al. 2008a, b). Stomach contents of strandings in North Cyprus were dominated by benthic prey items including bivalves and sponges (unpubl data). In comparison, the diet of juveniles caught in the Central Mediterranean were dominated by benthic prey items, including Malacostraca, Gastropoda, and Echinoidea, as well as pelagic prey items (Casale et al. 2008). In the western Mediterranean juveniles caught predominantly in neritic habitats had consumed both pelagic and benthic–demersal prey, including fish, pelagic tunicates, crustaceans, molluscs, and other invertebrates (Tomás et al. 2001). Whilst in the Northern Adriatic, small juveniles that would have previously been considered oceanic in size had diets dominated by benthic items such as anemones, crustaceans, and molluscs (Lazar et al. 2008a, b).
Mediterranean juveniles appear to follow alternative life-history patterns to those in other ocean basins and intra-population differences in habitat use are also reported (Casale et al. 2008, 2015). In regions, such as Amvrakikos Gulf (Greece) and Cyprus, most individuals found in coastal neritic habitats are larger (mean CCL: 0.67 and 0.65 m, respectively; Rees et al. 2013; Snape et al. 2013). This supports the traditional ontogenetic life-history model of a distinct shift in preference from oceanic to neritic habitat use with increased size (Musick and Limpus 1997). This traditional life-history model is challenged on the Tunisian Plateau, Northern Adriatic Sea, and in the western Mediterranean, where juveniles as small as 0.25 m in length (notch-to-tip, Bolten 1999) start to utilise both neritic and oceanic habitats interchangeably, and are therefore, susceptible to threats in both habitats (Tomás et al. 2001; Casale et al. 2008; Lazar et al. 2008a, 2011).
In the Mediterranean Sea, bycatch is one of the key threats to marine turtles resulting in high levels of mortality in both neritic and oceanic habitats (conservatively 44,000 deaths per year, Casale 2011; Casale et al. 2018). The susceptibility of juvenile loggerhead turtles to anthropogenic threats differs with region due to heterogeneity in fishing effort as well as due to differences in habitat use by turtles (e.g. Cardona et al. 2009; Casale 2011). To loggerhead turtles foraging in neritic habitats, the threat comes from small-scale fisheries using nets (trammel and gill) and bottom-set longlines, whilst interactions with pelagic longline are more common for oceanic foragers (Casale 2011). Region and habitat use will also likely affect the susceptibility of marine turtles to other anthropogenic threats such as the ingestion of debris and chemical pollution (Franzellitti et al. 2004; Casale et al. 2008, 2016). Understanding the foraging habitats used by all individuals within and among populations is necessary to assess threats and implement appropriate management approaches. Therefore, using SIA of stranded, incidentally and directly captured juveniles, this study aims to assess the foraging ecology of juvenile loggerhead turtles from a range of foraging regions in the Mediterranean Sea, to determine if foraging ecology differs with region, sex, and size.
Materials and methods
Sample collection
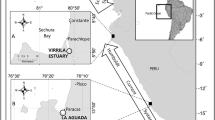
Carapace costal scute samples were obtained from dead incidentally captured juvenile loggerhead turtles found in the NE Adriatic (Croatia and Slovenia, n = 52) and Central Mediterranean (Lampedusa, n = 36, Fig. 1) between 2001 and 2006. These turtles were captured by trawl, longline, or static net fishing gear. Scute samples were taken by scalpel from the second or third costal scute. The exact location of the incidentally captured individuals in the Central Mediterranean is unknown as turtles were collected from fishers on the Tunisian continental shelf and landed in Lampedusa. In the North Levantine basin (North Cyprus), 228 juveniles were stranded dead or incidentally captured (dead and alive in trammel nets) between 2012 and 2018. Of these, 65 were sampled for epidermis tissue (< 0.25 cm2, Fig. 1) from the trailing edge of the fore flipper on the third membrane or the shoulder (between the neck and fore flipper). Epidermis tissue samples were also collected from the third membrane from the trailing edge of the fore flipper of live-captured juveniles foraging in the East Ionian (Amvrakikos Gulf, Greece, n = 18, Fig. 1) in 2017 (see Rees et al. 2013 for details on the capture method). Skin samples were taken by scalpel and only the epidermis tissue was used in the analysis (dermis tissue was removed in the laboratory). Until required for analysis, scute samples were air-dried then frozen and epidermis samples were stored in ethanol (90% and 70% ethanol in East Ionian and North Levantine basin, respectively) at room temperature.
a Locations of juvenile loggerhead turtles sampled in the Central Mediterranean (CMed, open circle – at sea locations are unknown), East Ionian (EIon, black circle), NE Adriatic (NEA, red circles), and North Levantine basin (NL, blue circles). The location where juveniles were sampled is shown in (b) for the NE Adriatic and (c) for the North Levantine basin. 200 m isobath is indicated (grey line). Artwork inset of a loggerhead turtle foraging
Curved carapace length (CCL) was measured with a flexible measuring tape as an indicator of body size. CCL measurements in the Central Mediterranean, East Ionian, and NE Adriatic were notch-to-tip, whilst CCL measurements in the North Levantine basin were notch-to-notch (Bolten 1999; for conversion of notch-to-tip to notch-to-notch values, see Appendix S1 in the Online Resource). Individuals were considered juvenile if CCL < 0.80 m, which is the rookery-weighted mean size at sexual maturity for Mediterranean loggerhead turtles, and was selected as genetics suggest mixed stocks in the foraging grounds (Casale et al. 2005, 2018; Casale and Heppell 2016). For dead juveniles, sex was determined by gross morphology and/or histology of the gonads (Casale et al. 2006; Lazar et al. 2008b), whilst sex was unknown for live-caught and live-bycaught juveniles in the East Ionian and North Levantine basin, respectively, as sex is not usually dimorphic at juvenile stages and gross morphology of the gonads could not be performed.
Stable isotope analysis
Scute samples were cleaned to remove epibionts and rinsed with ethanol. Both scute and epidermis samples were rinsed with deionized water, soaked for 24 h, and dried at 60 ˚C for 48 h. Approximately 0.70 mg (± 0.10 mg) of sample was weighed into sterilised tin capsules. Epidermis samples did not undergo lipid extraction and did not require a lipid correction factor as evaluated by the C:N ratio (mean:3.44, range: 3.18–3.78, Post et al. 2007). Samples were analysed on a Thermoquest EA1110 elemental analyser linked to a Sercon2020 stable isotope ratio mass spectrometer running in continuous flow mode (conducted by Elemtex Ltd, UK laboratory). Isotope ratios are expressed as conventional delta (δ) values in parts per thousand (‰) using the following equation: δX = [( Rsample / Rstandard) – 1] × 1000, where X is 13C or 15 N. Rsample and Rstandard are the corresponding ratios of the heavier to the lighter isotope (i.e., 13C/12C, 15 N/14 N) in the sample and international standard, respectively. The international standard for 13C and 15 N is Vienna Pee Dee Belemnite and atmospheric nitrogen (AIR), respectively.
All analyses were performed with the software R 3.5.1 (R Core Team 2018), and for statistical tests, the significance level used was α = 0.05. To determine if region affects δ13C and δ15N values, whilst taking size into account, an Analysis of Covariance was performed. To determine if sex affected stable isotope ratios an Analysis of Variance (ANOVA) was performed, whilst a General Additive Model (GAM) was performed using the R package ‘mgcv’ (Wood 2011) to determine if size affected δ13C and δ15N values, with size set as a smooth term.
The isotopic niche width of individuals grouped by region or sex was calculated using the R package ‘SIBER’ (Stable Isotope Bayesian Ellipses in R, Jackson et al. 2011). Maximum-likelihood standard ellipses were obtained by Bayesian inference containing 40% of the data (SEA) and small samples sizes were corrected for (SEAc). Isotope niche overlap among each group was calculated as the proportion of the non-overlapping area of the two ellipses. See Jackson et al. (2011) for details on these methods.
The time between death and sampling is unknown for stranded individuals; however, decomposition is not thought to affect δ13C and δ15N values of loggerhead turtle epidermis (Payo-Payo et al. 2013). We compared the stable isotope ratios of juveniles with different decomposition states (categorised as: alive, fresh dead, moderately decomposed, severely decomposed, and skeleton) and found no significant differences, and therefore, for further analysis, individuals were not analysed seperately based on decomposition state. Stable isotope ratios of stranded and incidentally captured juveniles from the North Levantine basin did not differ isotopically and therefore from herein were treated as one group and referred to as stranded unless specified otherwise (for details on these analyses, see Appendix S2 in the Online Resource). To determine temporal shifts in baseline ratios for each region, stable isotope ratios were compared across the sampling periods using ANOVAs. To determine monthly differences in the δ13C and δ15N values of epidermis samples from the North Levantine basin, Generalised Additive Mixed Models (GAMM) were used in the R package ‘mgcv’ (Wood 2011). The GAMM used a cyclic smoothing spline to account for the annual cyclic trend.
Results
In total, tissue from 171 juveniles were analysed from the Central Mediterranean, East Ionian, NE Adriatic, and North Levantine basin (Table 1). δ13C values ranged from − 19.32 to − 12.76‰ (mean ± SD = − 16.60 ± 1.34‰, n = 171) and δ15N values ranged from 3.94 to 13.71‰ (mean ± SD = 8.03 ± 2.14‰, n = 171).
For 88 individuals, replicate scute samples were analysed, but no significant difference was found between replicates for δ13C and δ15N values (Wilcoxon signed-rank test, δ13C: V = 1657, Z = − 1.10, P = 0.27, n = 88; δ15N: V = 1547, Z = − 1.40, P = 0.16, n = 18), and as a result, the mean value was used for further analysis. The results were found to be insensitive to the isotope analytical uncertainties (for details of this analysis, see Appendix S5 in the Online Resource).
To determine temporal shifts in baseline ratios for each region, stable isotope ratios were compared across the sampling periods. Neither δ13C nor δ15N values differed with year in all regions (ANOVA, Central Mediterranean: δ13C: F(2,19) = 0.15, P = 0.86, δ15N: F(2,19) = 0.90, P = 0.42, n = 36; NE Adriatic: δ13C: F(4,46) = 0.13, P = 0.97, δ15N: F(4,46) = 0.10, P = 0.98, n = 52; North Levantine basin: δ13C: F(6,58) = 1.28, P = 0.28, δ15N: F(6,58) = 2.13, P = 0.06, n = 65, see Appendix S3 in the Online Resource). These results were found to be insensitive to the isotope analytical uncertainties; however, note the higher uncertainties (resulting in a lower performance consistency for nitrogen) for the North Levantine basin (for details of this analysis, see Appendix S5). East Ionian samples were not included in this analysis as all samples were collected in 2017 only. A significant difference was seen in δ13C values with month for the samples collected in the North Levantine basin (GAMM: F = 1.53, edf = 2.32, p < 0.002, R2 = 0.17, n = 4) with higher δ13C values in the summer months (Fig S4.4). No difference was seen in δ15N values with month (GAMM: F = 1.53, edf = 2.32, p < 0.002, R2 = 0.17, n = 4, Fig S4.4). These results were found to be insensitive to the isotope analytical uncertainties (for details of this analysis see Appendix S5).
Inter-region differences
A significant difference was seen in δ13C values among regions (ANOVA, F(3,167) = 80.49, P < 0.001, n = 171) and a post hoc Tukey’s Honest Significant Difference test showed this was due to the δ13C values of all regions differing with juveniles from Central Mediterranean having the lowest values (P < 0.001, Fig. 2). When body size was taken into account, region continued to affect δ13C values (ANCOVA, F(3,163) = 81.80, P < 0.001, n = 171). A significant difference was seen in δ15N values among regions (ANOVA: F(3,167) = 59.99, P < 0.001, n = 171) and a post hoc Tukey’s Honest Significant difference test shows that this was due to the δ15N values of all regions differing with juveniles from NE Adriatic having the highest values (P < 0.001, Fig. 2). When body size was taken into account, region continued to affect δ15N values (ANCOVA, F(3,163) = 63.78, P < 0.001, n = 171). These results were found to be insensitive to the isotope analytical uncertainties (for details of this analysis, see Appendix S5). SIBER results show the isotope niche of Central Mediterranean and NE Adriatic juveniles which are distinct as their overlaps were null, whilst juveniles in the North Levantine basin slightly overlapped with East Ionian juveniles (Table 2; Fig. 2).
a δ13C values and b δ15N values of juvenile loggerhead turtles sampled in the Central Mediterranean (CMed, grey, n = 36), East Ionian (EIon, black, n = 18), NE Adriatic (NEA, red, n = 52), and the North Levantine basin (NL, blue, n = 65). Midline = median, box = interquartile range, whiskers = 5 and 95 percentiles. c Bivariate plot of δ13C and δ15N values showing the isotope niche coloured by region. Ellipses = Standard ellipse area corrected for small sample size (SEAc) created by SIBER
Sex-specific differences
In total, sex was determined for 36 juveniles in the Central Mediterranean (F = 16, M = 20), 45 in the NE Adriatic (F = 33, M = 12), and 37 in the North Levantine basin (F = 21, M = 16). Neither δ13C nor δ15N values differed between female and male juvenile loggerhead turtles within each region (ANOVA, Central Mediterranean: δ13C: F(1,34) = 0.87, P = 0.36, δ15N: F(1,34) = 1.92, P = 0.17, n = 36; NE Adriatic: δ13C: F(1,43) = 3.15, P = 0.08, δ15N: F(1,43) = 0.10, P = 0.76, n = 45; North Levantine basin: δ13C: F(1,35) = 0.02, P = 0.90, δ15N: F(1,35) = 1.72, P = 0.20, n = 37, Fig. 3). These results were found to be insensitive to the isotope analytical uncertainties (for details of this analysis, see Appendix S5). SIBER results show that the isotope niches of females and males are not distinct in any region (Table 2; Fig. 3).
a δ13C and b δ15N values of female and male juvenile loggerhead turtles sampled in the Central Mediterranean (grey, n: F = 16, M = 20), NE Adriatic (red, n: F = 33, M = 12), and North Levantine basin (blue, n: F = 21, M = 16). F: Female, M: male. Midline = median, box = interquartile range, whiskers = 5 and 95 percentiles. c Bivariate plot of δ13C and δ15N values showing the isotope niche of females (solid lines) and males (dashed lines) coloured by region. Ellipses = Standard ellipse area corrected for small sample size (SEAc) created by SIBER. Sex was unknown for East Ionian juveniles as they were live-caught
Size differences
A full range of juvenile sizes were sampled from 0.12 to 0.79 m (mean CCL = 0.54 m). Size significantly differed among regions (ANOVA, F(3,167)=40.8, P < 0.001, n = 171). A post hoc Tukey’s Honest Significant Difference test showed that juveniles sampled from the East Ionian and the North Levantine basin were significantly larger than juveniles from the Central Mediterranean and NE Adriatic (P < 0.001). The δ13C values of juvenile loggerhead turtles were not affected by size in any region (GAM, P > 0.05, Fig. 4). The δ15N values were not affected by size in the Central Mediterranean or East Ionian, whilst larger individuals had higher δ15N values in the NE Adriatic (GAM, F = 7.24, P = 0.009) and the North Levantine basin (F = 3.05, P = 0.04, Fig. 4). These results were found to be insensitive to the isotope analytical uncertainties (for details of this analysis, see Appendix S5).
Summary of the influence of curved carapace length (CCL) on δ13C values (left column) and δ15N values (right column) of juvenile loggerhead turtles sampled in the (a, b) Central Mediterranean, (c, d) NE Adriatic, (e, f) North Levantine basin, and (g, h) East Ionian. Solid line represents mean isotope ratio response and shaded region represents ± standard error. Edf estimated degrees of freedom, F F-statistic, p significance. Note different x-axis for East Ionian plots
Discussion
The results highlight the ecological complexity of Mediterranean juvenile loggerhead turtles and demonstrate the benefits of conducting SIA on opportunistically obtained juveniles for understanding the foraging ecology of marine vertebrates. Regional differences are observed in stable isotope ratios, and intra-regional variation occurs with size but not sex, therefore supporting a requirement for site specific management approaches.
Inter-region differences
Differences in stable isotope ratios among regions are more likely due to baseline variations in δ13C and δ15N values rather than geographical differences in foraging ecology. The Central Mediterranean sampling region is offshore (~ 160 km), surrounded by both neritic and oceanic habitats, from which loggerhead turtles forage (Casale et al. 2008). Although on the continental shelf, food chains in this offshore region are likely supported by phytoplankton, which have lower δ13C values in comparison to productive benthic and near-shore regions with food chains supported by algae and seagrass (DeNiro and Epstein, 1978; Graham et al. 2010). This likely explains why juveniles foraging in the Central Mediterranean have lower δ13C values than juveniles foraging in the East Ionian, NE Adriatic, and North Levantine basin, which are likely foraging predominantly in neritic habitats. This trend has been observed in several loggerhead turtle populations (e.g. Hatase et al. 2002; Eder et al. 2012). Although a stepwise enrichment in δ13C values can be seen with each subsequent trophic level, it is unlikely juveniles foraging in the Central Mediterranean are foraging at lower trophic levels than the other regions as they do not have lower δ15N values (except in comparison to the NE Adriatic, DeNiro and Epstein 1978; Minagawa and Wada 1984; France and Peters 1997).
High δ15N values have been previously reported for loggerhead turtles foraging in the NE Adriatic and have been attributed to the extensive influence of highly enriched 15 N agricultural run-off and anthropogenic waste from major river systems (Degobbis and Gilmartin 1990; Zbinden et al. 2011; Cardona et al. 2014; Haywood et al. 2020). In comparison, relatively low baseline δ15N values are seen across the eastern Mediterranean basin, which includes the North Levantine basin and the Central Mediterranean and is most likely due to high levels of N2fixation (Pantoja et al. 2002).
Differences in sampling methods among the geographical regions may also bias the results. For example, the sampling area of each geographical region differs substantially with the East Ionian individuals sampled from a discrete neritic site in the Amvrakikos Gulf with limited foraging options (max depth 65 m, Rees et al. 2013), and the NEA Adriatic and North Levantine basin were sampled in a relatively discrete area, whereas a large area was fished in the Central Mediterranean where juveniles likely had access to multiple foraging habitats (Casale et al. 2008). Sampling method differed with region with individuals in the East Ionian live caught in targeted foraging grounds, individuals from Central Mediterranean, and NE Adriatic incidentally captured, whilst individuals from the North Levantine basin were incidentally captured or stranded. The cause of stranding was often unidentified and the location in which the turtle died was unknown.
In addition, incidentally captured individuals were caught in different fishing gears dependent on the geographical region. In the North Levantine basin, individuals were incidentally captured in trammel nets, therefore, incidentally targeting neritic foragers, whilst individuals caught in the Central Mediterranean and NEA Adriatic were caught by trawl, longline, or static net fishing gear, and in turn sampling either benthic or pelagic habitats. The SIBER results show that juveniles in the East Ionian have the narrowest isotopic niche, which may be due to limited foraging options or due to the small sample size (although small sample sizes were corrected for). Juveniles in the Central Mediterranean and NE Adriatic have relatively small isotope niche widths, whilst juveniles foraging in the North Levantine basin had the largest. The larger isotope niche width seen for North Levantine basin juveniles could suggest that they are foraging on a larger range of prey or are using a larger range of habitats. The mean size (CCL) at sexual maturity for loggerhead turtles in the Mediterranean is considered 0.80 m (Casale and Heppell 2016), but females nesting in North Cyprus and foraging in other regions of the Mediterranean can be considerably smaller (minimum recorded was 0.59 m, unpublished data). Therefore, some of the individuals sampled in the North Levantine basin and assigned as juveniles may in fact nest in North Cyprus, but forage in other areas of the Mediterranean, resulting in a large isotope niche width for the North Levantine basin group.
It should be noted that in this study, two tissues types, scute and epidermis, were used. The isotope ratios represent a time intergrated diet with each tissue type respresenting different time frames of dietary information due to differences in the metabolic turnover rate (Peterson & Fry 1987). Epidermis incorporates dietary information over several months (Reich et al. 2008), whilst scute represents a longer time frame (e.g. Vander Zanden et al. 2010). This is not an issue when studying adults as they are known to show high foraging site fidelity and, therefore, have relatively constant isotope ratios through their scutes. However, this can be a limitation for juvenile loggerhead turtles in the Mediterranean as although some remain in distinct grounds, others have been found to shift habitats relativley frequently (e.g. Cardona et al. 2005, 2009; Casale et al. 2007, 2012; Eckert et al. 2008). Epidermis samples from the North Levantine basin show a seasonal change in carbon isotope ratios demonstrating a potential habitat or dietary shift through the year and may explain the larger isotope niche reported in this region. Scute samples analysed from Central Mediterranean and NE Adriatic juveniles may, therefore, represent a combination of several habitats and prey items. As juveniles from these regions had distinct isotope ratios, relatively small isotope niche widths, and isotope ratios that match the current knowledge about the isoscape of the Mediterranean, it suggests that even if these individuals are frequenting several habitats, they are likely remaining in the same geographical region.
Although tissue–tissue conversion equations enable isotopes ratios from different tissue types to be compared, they should be used with caution as there are numerous factors that can influence isotopic differentiation between tissues. Therefore, we support the previous reccomendations that a standardised tissue type should be used enabling direct comparisons between studies especially when investigating juveniles (see Haywood et al. 2019 and citations within).
Sex-specific differences
Differences between resource use of female and male adults might be expected due to various evolutionary and energetic pressures related to reproduction (Pajuelo et al. 2016), although differences may not be evident until they have reached sexual maturity. No difference in the foraging ecology of female and male loggerhead turtles has been documented previously in the Mediterranean or other ocean basins (Tomás et al. 2001; Seney and Musick 2007; Schofield et al. 2010, 2013; Pajuelo et al. 2012; Thomson et al. 2012; Casale et al. 2013; Hays et al. 2014b). The absence of sex-specific differences in stable isotope ratios, the high overlap in isotope niche, and the similar sex ratios at each sample site suggest juvenile males and females exploit similar prey items and inhabit similar areas. With both sexes utilising the same resources, their susceptibility to fisheries threats will likely be similar. This supports the findings of an unbiased sex ratio of bycaught juveniles in the Mediterranean Sea previously reported by Casale et al. (2006).
Size differences
Higher δ13C and δ15N values with size have been previously reported for both juvenile and adult loggerhead turtles in the Mediterranean and other ocean basins (Godley et al. 1998; Hatase et al. 2002; Pajuelo et al. 2010; Eder et al. 2012; Goodman Hall et al. 2015; Ramirez et al. 2015; Blasi et al. 2018), but this is not always the case (Wallace et al. 2009; Clusa et al. 2016). This suggests that shifts in habitat use or diet with size are not obligate, and a relaxed life-history model has been previously reported in the Mediterranean Sea (Casale et al. 2008). Higher δ15N values in larger juveniles could suggest that larger individuals are foraging in more neritic habitats which have comparatively higher baseline δ13C and δ15N values compared to oceanic habitats (Hatase et al. 2002; McClellan et al. 2010; Ramirez et al. 2015). This isotope ratio and size trend is well reported for populations undertaking oceanic–neritic ontogenetic shifts during the juvenile life stage (Snover et al. 2010; Ramirez et al. 2015; Turner Tomaszewicz et al. 2017).
However, it is very likely the loggerhead foraging grounds in the NE Adriatic and North Levantine basin are on the continental shelf, and therefore, differences in oceanic and neritic foraging habitat are less likely than differences in epi-pelagic verses benthic prey consumption. Due to trophic fractionation, higher trophic prey items have higher δ15N values (DeNiro and Epstein 1978; Minagawa and Wada 1984; France and Peters 1997; Belicka et al. 2012). Improvement in diving capacity (depth and duration) as well as larger heads, larger gape size, and, therefore, higher bite force with size (Salmon et al. 2004; Marshall et al. 2012) means previously inaccessible higher trophic fauna, such as large molluscs, crustaceans, and fish, become available to larger individuals (Seney and Musick 2007, Casale et al. 2008; Goodman Hall et al. 2015; Blasi et al. 2018). This would explain the size trend observed in this study for juveniles foraging in the North Levantine basin and NE Adriatic, whilst the small size range in East Ionian turtles may explain why no size effects were reported. With trammel and gill nets, as well as bottom-set longlines, being the highest cause of bycatch in neritic habitats (Casale 2011), juvenile loggerheads may become more susceptible to neritic fishing gears in these regions as they grow, as they may be foraging on more neritic prey items.
In contrast, in the Central Mediterranean, Casale et al. (2008) reported benthic and epi-pelagic prey was commonly consumed in both neritic and oceanic individuals of all size classes. This not only suggests foraging throughout the water column, but the use of both neritic and oceanic habitats interchangeably (Casale et al. 2008). This was also found in the western Mediterranean with no differences in isotope ratios reported for juveniles caught in neritic or oceanic habitats or between individuals of different sizes suggesting the consumption of similar dietary items (Revelles et al. 2007). Hence, isotope ratios of an individual could incorporate baseline isotope ratios of both neritic and oceanic habitats and would explain why no size trend was seen in juveniles sampled from the Central Mediterranean in this study or for juveniles sampled in southern Italy by Clusa et al. (2016). With bycatch in the Central Mediterranean spanning both the neritic and oceanic habitats (as emphasised by samples in this region collected from trawl, pelagic longline, and static net fishing gear), the results from this study suggest juveniles in the Central Mediterranean may be bycaught in both habitats throughout their size range.
Implications for conservation
SIA has been used globally to demonstrate size-related differences in habitat use for loggerhead turtles and in turn highlighting the need for conservation management to consider population sub-groups (e.g. Hatase et al. 2002; McClellan et al. 2010; Snover et al. 2010; Thomson et al. 2012; Ramirez et al. 2015; Turner Tomaszewicz et al. 2017). In neritic habitats, such as in the North Levantine basin, bottom-set fishing gear is most common (Casale 2011). With larger individuals potentially consuming a more benthic dominated diet, this may increase the probability of bycatch in this size class. This has been previously reported by Snape et al. (2013) who found that mostly larger individuals were bycaught in Cyprus and attributed most strandings to small-scale fisheries using demersal gears, which could cause a shift in population dynamics. In the water of eastern mainland Spain, juveniles appear to extensively use the continental shelf where their susceptibility to neritic fishing gears is high (Cardona et al. 2009). In comparison, with juveniles of all sizes utilising both neritic and oceanic habitats in the Central Mediterranean, interactions with both bottom-set gears and pelagic longlines are likely. This is supported by research by Clusa et al. (2016) that showed isotope and genetic markers of Atlantic and Mediterranean juveniles in the western and central Mediterranean differed with region but not between pelagic or neritic fishing gears. This suggests that these juveniles are using both habitats interchangeably, and it was concluded that in these areas of the Mediterranean, the impact of turtle bycatch depends on the geographic distribution of the fishing effort rather than the fishing type (Clusa et al. 2016) unlike in the North Levantine basin. With different foraging strategies used in different regions of the Mediterranean, region-specific management approaches are required, dependent on whether management of fishing gear or fishing location would be most beneficial.
Surface currents in the global oceans are thought to passively disperse loggerhead turtle hatchlings to the foraging grounds that they continue to return to throughout their life time (Hays et al. 2010; Putman et al. 2012; Scott et al. 2012; Casale and Mariani 2014). The distinct isotope niche of juveniles in each geographical region in this study suggests a limited exchange of individuals between these areas and, therefore, supports the hypothesis that large juveniles remain in the same geographical region which they passively drifted to. With juveniles likely remaining in the same geographical region, the susceptibility to fisheries interactions will differ as fishing effort and fishing gear is not spatially homogenous across the Mediterranean (Casale 2011 and citations within). Marine turtles face other anthropological threats and the level of these also differs with region and habitat use (see review by Casale et al. (2018)). For example, in the north Adriatic, loggerhead turtles have high levels of heavy metals (Franzellitti et al. 2004), whilst low levels were reported in Cyprus turtles 20 years ago (Godley et al. 1999). Individuals with a higher trophic position are thought to have heavier burdens of pollutants due to diet-related bioaccumulation (Mckenzie et al. 1999). In addition, Central Mediterranean loggerhead turtles caught in pelagic habitats have higher rates of debris ingestion compared to those in neritic habitats (Casale et al. 2016). Therefore, for conservation management to be successful, the spatial and foraging ecology of marine turtles must be considered.
To further enhance our understanding of the complexities of loggerhead turtle foraging ecology globally, we support the recommendations to (1) use additional forensic markers or complementary techniques to provide greater power of inference of dietary estimations and geographical differences, (2) for standardised methods to be used to allow comparisons across studies, and (3) for the collaboration and combining of datasets at a global scale (as reviewed in Haywood et al. (2019) and citations within).
Conclusions
This study highlights the use of stable isotope analysis to better understand the foraging ecology of marine vertebrates. For juvenile loggerhead turtles in the Mediterranean Sea, differences in foraging ecology do not occur between sexes, but do occur among geographical regions and with size. Differences in stable isotope ratios among geographical regions are likely due to the different habitats used by each population, with individuals in the Central Mediterranean using more oceanic habitats than the other populations. Susceptibility of these regions to different fisheries will, therefore, be likely and should be considered in future-management strategies. Size differences were region-dependent with no differences reported in regions where oceanic and neritic habitats were available suggesting juveniles in these regions will be bycaught by multiple fishing gears throughout their size range. In regions with only neritic habitats, differences were attributed to larger individuals exploiting different prey and suggest that individuals of different sizes may play different roles in the ecosystem and, in turn, become more susceptible to neritic fishing gears as they grow. These results confirm the necessity of implementing region as well as habitat-specific management approaches.
Data availability
The data sets collected and analysed during the current study are available from the corresponding author on reasonable request.
References
Araújo MS, Bolnick DI, Layman CA (2011) The ecological causes of individual specialisation. Ecol Lett 14(9):948–958
Arendt MD, Segars AL, Byrd JI, Boynton J, Schwenter JA, Whitaker JD, Parker L (2012) Migration, distribution, and diving behavior of adult male loggerhead sea turtles (Caretta caretta) following dispersal from a major breeding aggregation in the Western North Atlantic. Mar Biol 159(1):113–125
Belicka LL, Burkholder D, Fourqurean W, Heithaus MR, Macko SA, Jaffé R (2012) Stable isotope and fatty acid biomarkers of seagrass, epiphytic, and algal organic matter to consumers in a pristine seagrass ecosystem. Mar Freshw Res 63(11):1085–1097
Bernhardt JR, Leslie HM (2013) Resilience to climate change in coastal marine ecosystems. Annu Rev Mar Sci 5:371–392
Bird CS, Veríssimo A, Magozzi S, Abrantes KG, Aguilar A, Al-Reasi H, Barnett A, Bethea DM, Biais G, Borrell A, Bouchoucha M (2018) A global perspective on the trophic geography of sharks. Nat Ecol Evol 2(2):299
Blasi MF, Tomassini L, Gelippi M, Careddu G, Insacco G, Polunin NVC (2018) Assessing resource use patterns of Mediterranean loggerhead sea turtles Caretta caretta (Linnaeus, 1758) through stable isotope analysis. Eur Zool J 85(1):71–87
Bolten AB (1999) Techniques for measuring sea turtles. In: Eckert KL, Bjorndal KA, Abreu-Grobois FA, Donnelly M (eds) Research and Management Techniques for the Conservation of Sea Turtles. IUCN/SSC Marine Turtle Specialist Group 4, Washington, DC, pp 110–114
Bolten AB (2003) Variation in sea turtle life history patterns: neritic vs. oceanic developmental stages. In: Lutz PL, Musick JA, Wyneken J (eds) The biology of sea turtles, vol 2. CRC Press, Boca Raton, pp 243–257
Cardona L, Revelles M, Carreras C, San Félix M, Gazo M, Aguilar A (2005) Western Mediterranean immature loggerhead turtles: habitat use in spring and summer assessed through satellite tracking and aerial surveys. Mar Biol 147(3):583–591
Cardona L, Revelles M, Parga ML, Tomás J, Aguilar A, Alegre F, Raga A, Ferrer X (2009) Habitat use by loggerhead sea turtles Caretta caretta off the coast of eastern Spain results in a high vulnerability to neritic fishing gear. Mar Biol 156(12):2621–2630
Cardona L, De Quevedo IÁ, Borrell A, Aguilar A (2012) Massive consumption of gelatinous plankton by Mediterranean apex predators. PLoS ONE 7(3):e31329
Cardona L, Clusa M, Eder E, Demetropoulos A, Margaritoulis D, Rees AF, Hamza AA, Khalil M, Levy Y, Türkozan O, Marín I (2014) Distribution patterns and foraging ground productivity determine clutch size in Mediterranean loggerhead turtles. Mar Ecol Prog Ser 497:229–241
Cardona L, Martínez-Iñigo L, Mateo R, González-Solís J (2015) The role of sardine as prey for pelagic predators in the western Mediterranean Sea assessed using stable isotopes and fatty acids. Mar Ecol Prog Ser 531:1–14
Casale P (2011) Sea turtle by-catch in the Mediterranean. Fish Fish 12(3):299–316
Casale P, Heppell SS (2016) How much sea turtle bycatch is too much? A stationary age distribution model for simulating population abundance and potential biological removal in the Mediterranean. Endang Spec Res 29(3):239–254
Casale P, Freggi D, Basso R, Argano R (2005) Size at male maturity, sexing methods and adult sex ratio in loggerhead turtles (Caretta caretta) from Italian waters investigated through tail measurements. Herpetol J 15(3):145–148
Casale P, Lazar B, Pont S, Tomás J, Zizzo N, Alegre F, Badillo J, Di Summa A, Freggi D, Lackovic G, Raga JA (2006) Sex ratios of juvenile loggerhead sea turtles Caretta caretta in the Mediterranean Sea. Mar Ecol Prog Ser 324:281–285
Casale P, Freggi D, Basso R, Vallini C, Argano R (2007) A model of area fidelity, nomadism, and distribution patterns of loggerhead sea turtles (Caretta caretta) in the Mediterranean Sea. Mar Biol 152(5):1039–1049
Casale P, Abbate G, Freggi D, Conte N, Oliverio M, Argano R (2008) Foraging ecology of loggerhead sea turtles Caretta caretta in the central Mediterranean Sea: evidence for a relaxed life history model. Mar Ecol Prog Ser 372:265–276
Casale P, Broderick AC, Freggi D, Mencacci R, Fuller WJ, Godley BJ, Luschi P (2012) Long-term residence of juvenile loggerhead turtles to foraging grounds: a potential conservation hotspot in the Mediterranean. Aquatic Conserv 22(2):144–154
Casale P, Freggi D, Cina A, Rocco M (2013) Spatio-temporal distribution and migration of adult male loggerhead sea turtles (Caretta caretta) in the Mediterranean Sea: further evidence of the importance of neritic habitats off North Africa. Mar Biol 160(3):703–718
Casale P, Mariani P (2014) The first ‘lost year’ of Mediterranean sea turtles: dispersal patterns indicate subregional management units for conservation. Mar Ecol Prog Ser 498:263–274
Casale P, Freggi D, Furii G, Vallini C, Salvemini P, Deflorio M, Totaro G, Raimondi S, Fortuna C, Godley BJ (2015) Annual survival probabilities of juvenile loggerhead sea turtles indicate high anthropogenic impact on Mediterranean populations. Aquatic Conserv 25(5):690–700
Casale P, Freggi D, Paduano V, Oliverio M (2016) Biases and best approaches for assessing debris ingestion in sea turtles, with a case study in the Mediterranean. Mar Pollut Bull 110(1):238–249
Casale P, Broderick AC, Camiñas JA, Cardona L, Carreras C, Demetropoulos A, Fuller WJ, Godley BJ, Hochscheid S, Kaska Y, Lazar B (2018) Mediterranean Sea turtles: current knowledge and priorities for conservation and research. Endang Spec Res 36:229–267
Chapin FS, Sala OE, Huber-Sannwald E, Iii FS (2001) Global biodiversity in a changing environment: scenarios for the 21st century (Vol. 152). Springer Science & Business Media.
Clusa M, Carreras C, Pascual M, Gaughran SJ, Piovano S, Avolio D, Ollano G, Fernández G, Tomás J, Raga JA, Aguilar A (2016) Potential bycatch impact on distinct sea turtle populations is dependent on fishing ground rather than gear type in the Mediterranean Sea. Mar Biol 163(5):122
Degobbis D, Gilmartin M (1990) Nitrogen, phosphorus, and biogenic silicon budgets for the northern Adriatic Sea. Oceanol Acta 13(1):31–45
DeNiro MJ, Epstein S (1978) Influence of diet on the distribution of carbon isotopes in animals. Geochim Cosmochim Acta 42(5):495–506
Duffy DC, Jackson S (1986) Diet studies of seabirds: a review of methods. Colon Waterbirds 9(1):1–17
Eckert SA, Moore JE, Dunn DC, van Buiten RS, Eckert KL, Halpin PN (2008) Modeling loggerhead turtle movement in the Mediterranean: importance of body size and oceanography. Ecol Appl 8(2):290–308
Eder E, Ceballos A, Martins S, Pérez-García H, Marín I, Marco A, Cardona L (2012) Foraging dichotomy in loggerhead sea turtles Caretta caretta off northwestern Africa. Mar Ecol Prog Ser 470:113–122
Figgener C, Bernardo J, Plotkin PT (2019a) MarTurtSI, a global database of stable isotope analyses of marine turtles. Scientific Data 6(1):16
Figgener C, Bernardo J, Plotkin PT (2019b) Beyond trophic morphology: stable isotopes reveal ubiquitous versatility in marine turtle trophic ecology. Biol Rev Camb Philos Soc 94(6):1947–1973
France RL, Peters RH (1997) Ecosystem differences in the trophic enrichment of 13C in aquatic food webs. Can J Fish Aquat Sci 54(6):1255–1258
Franzellitti S, Locatelli C, Gerosa G, Vallini C, Fabbri E (2004) Heavy metals in tissues of loggerhead turtles (Caretta caretta) from the northwestern Adriatic Sea. Comp Biochem Physiol C Toxicol Pharmacol 138(2):187–194
Godley BJ, Thompson DR, Waldron S, Furness RW (1998) The trophic status of marine turtles as determined by stable isotope analysis. Mar Ecol Prog Ser 166:277–284
Godley BJ, Thompson DR, Furness RW (1999) Do heavy metal concentrations pose a threat to marine turtles from the Mediterranean Sea? Mar Pollut Bull 38(6):497–502
Godley BJ, Blumenthal JM, Broderick AC, Coyne MS, Godfrey MH, Hawkes LA, Witt MJ (2008) Satellite tracking of sea turtles: where have we been and where do we go next? Endang Spec Res 4(1–2):3–22
Goodman Hall A, Avens L, McNeill JB, Wallace B, Goshe LR (2015) Inferring long-term foraging trends of individual juvenile loggerhead sea turtles using stable isotopes. Mar Ecol Prog Ser 537:265–276
Graham BS, Koch PL, Newsome SD, McMahon KW, Aurioles D (2010) Using isoscapes to trace the movements and foraging behavior of top predators in oceanic ecosystems. In: West J, Bowen G, Dawson T, Tu K (eds) Isoscapes. Springer, Dordrecht, The Netherlands, pp 299–318
Hamann M, Godfrey MH, Seminoff JA, Arthur K, Barata PCR, Bjorndal KA, Bolten AB, Broderick AC, Campbell LM, Carreras C, Casale P (2010) Global research priorities for sea turtles: informing management and conservation in the 21st century. Endang Spec Res 11(3):245–269
Hatase H, Takai N, Matsuzawa Y, Sakamoto W, Omuta K, Goto K, Arai N, Fujiwara T (2002) Size-related differences in feeding habitat use of adult female loggerhead turtles Caretta caretta around Japan determined by stable isotope analyses and satellite telemetry. Mar Ecol Prog Ser 233:273–281
Hays GC, Fossette S, Katselidis KA, Mariani P, Schofield G (2010) Ontogenetic development of migration: Lagrangian drift trajectoriessuggest a new paradigm for sea turtles. J R Soc Interface 7(50):1319–1327. https://doi.org/10.1098/rsif.2010.0009
Hays GC, Christensen A, Fossette S, Schofield G, Talbot J, Mariani P (2014a) Route optimisation and solving Zermelo's navigation problem during long distance migration in cross flows. Ecol Lett 17(2):137–143
Hays GC, Mazaris AD, Schofield G (2014b) Different male vs female breeding periodicity helps mitigate offspring sex ratio skews in sea turtles. Front Mar Sci 1:43
Haywood JC, Fuller WJ, Godley BJ, Shutler JD, Widdicombe S, Broderick AC (2019) Global review and inventory: how stable isotopes are helping us understand ecology and inform conservation of marine turtles. Mar Ecol Prog Ser 613:217–245
Haywood JC, Fuller WJ, Godley BJ, Margaritoulis D, Shutler JD, Snape RTE, Widdicombe S, Zbinden JA, Broderick AC (2020) Spatial ecology of loggerhead turtles: Insights from stable isotope markers and satellite telemetry. Divers Distrib. https://doi.org/10.1111/ddi.13023
Jackson AL, Inger R, Parnell AC, Bearhop S (2011) Comparing isotopic niche widths among and within communities: SIBER–Stable Isotope Bayesian Ellipses in R. J Anim Ecol 80(3):595–602
Jiguet F, Gadot AS, Julliard R, Newson SE, Couvet D (2007) Climate envelope, life history traits and the resilience of birds facing global change. Glob Change Biol 13(8):1672–1684
Lazar B, Gračan R, Zavodnik D, Tvrtković N (2008a) Feeding ecology of “pelagic” loggerhead turtles, Caretta caretta, in the northern Adriatic Sea: proof of an early ontogenetic habitat shift: In: Kalb H, Rohde AS, Gayheart K, Shanker K (eds) Proceedings of the 25th Symposium on Sea Turtle Biology and Conservation. NOAA Technical Memorandum NMFS-SEFSC-582: pp:93
Lazar B, Lacković G, Casale P, Freggi D, Tvrtković N (2008) Histological validation of gonad gross morphology to sex juvenile loggerhead sea turtles (Caretta caretta). Herpetol J 18:137–140
Lazar B, Gračan R, Katić J, Zavodnik D, Jaklin A, Tvrtković N (2011) Loggerhead sea turtles (Caretta caretta) as bioturbators in neritic habitats: an insight through the analysis of benthic molluscs in the diet. Mar Ecol 32(1):65–74
Lewison RL, Crowder LB, Wallace BP, Moore JE, Cox T, Zydelis R, McDonald S, DiMatteo A, Dunn DC, Kot CY, Bjorkland R (2014) Global patterns of marine mammal, seabird, and sea turtle bycatch reveal taxa-specific and cumulative megafauna hotspots. Proc Natl Acad Sci USA 111(14):5271–5276
Luschi P, Mencacci R, Cerritelli G, Papetti L, Hochscheid S (2018) Large-scale movements in the oceanic environment identify important foraging areas for loggerheads in central Mediterranean Sea. Mar boil 165(1):4
Mansfield KL, Saba VS, Keinath JA, Musick JA (2009) Satellite tracking reveals a dichotomy in migration strategies among juvenile loggerhead turtles in the Northwest Atlantic. Mar Biol 156(12):2555
Marshall CD, Guzman A, Narazaki T, Sato K, Kane EA, Sterba-Boatwright BD (2012) The ontogenetic scaling of bite force and head size in loggerhead sea turtles (Caretta caretta): implications for durophagy in neritic, benthic habitats. J Exp Biol 215(23):4166–4174
McClellan CM, Read AJ (2007) Complexity and variation in loggerhead sea turtle life history. Biol Lett 3(6):592–594
McClellan CM, Braun-McNeill J, Avens L, Wallace BP, Read AJ (2010) Stable isotopes confirm a foraging dichotomy in juvenile loggerhead sea turtles. J Exp Mar Biol Ecol 387(1–2):44–51
McKenzie C, Godley BJ, Furness RW, Wells DE (1999) Concentrations and patterns of organochlorine contaminants in marine turtles from Mediterranean and Atlantic waters. Mar Environ Res 47(2):117–135
Minagawa M, Wada E (1984) Stepwise enrichment of 15N along food chains: further evidence and the relation between δ15N and animal age. Geochim Cosmochim Acta 48(5):1135–1140
Mingozzi T, Mencacci R, Cerritelli G, Giunchi D, Luschi P (2016) Living between widely separated areas: long-term monitoring of Mediterranean loggerhead turtles sheds light on cryptic aspects of females spatial ecology. J Exp Mar Biol Ecol 485:8–17
Montoya JP (2007) Natural abundance of 15N in marine planktonic ecosystems. In: Michener R, Lajtha K (eds) Stable isotopes in ecology and environmental science. Blackwell, Malden, pp 176–201
Musick JA, Limpus CJ (1997) Habitat utilization and migration in juvenile sea turtles. In: Lutz PL, Musick JA (eds) The biology of sea turtles. CRC Press, Boca Raton, pp 137–163
Newsome SD, Clementz MT, Koch PL (2010) Using stable isotope biogeochemistry to study marine mammal ecology. Mar Mamm Sci 26(3):509–572
Pajuelo M, Bjorndal KA, Alfaro-Shigueto J, Seminoff JA, Mangel JC, Bolten AB (2010) Stable isotope variation in loggerhead turtles reveals Pacific-Atlantic oceanographic differences. Mar Ecol Prog Ser 417:277–285
Pajuelo M, Bjorndal KA, Reich KJ, Arendt MD, Bolten AB (2012) Distribution of foraging habitats of male loggerhead turtles (Caretta caretta) as revealed by stable isotopes and satellite telemetry. Mar Biol 159(6):1255–1267
Pajuelo M, Bjorndal KA, Arendt MD, Foley AM, Schroeder BA, Witherington BE, Bolten AB (2016) Long-term resource use and foraging specialization in male loggerhead turtles. Mar Biol 163(11):235
Pantoja S, Repeta DJ, Sachs JP, Sigman DM (2002) Stable isotope constraints on the nitrogen cycle of the Mediterranean Sea water column. Deep Sea Res I 49(9):1609–1621
Payo-Payo A, Ruiz B, Cardona L, Borrell A (2013) Effect of tissue decomposition on stable isotope signatures of striped dolphins Stenella coeruleoalba and loggerhead sea turtles Caretta caretta. Aquat Biol 18(2):141–147
Peterson BJ, Fry B (1987) Stable isotopes in ecosystem studies. Annu Rev Ecol Syst 18(1):293–320
Post DM, Layman CA, Arrington DA, Takimoto G, Quattrochi J, Montana CG (2007) Getting to the fat of the matter: models, methods and assumptions for dealing with lipids in stable isotope analyses. Oecologia 152(1):179–189
Putman NF, Scott R, Verley P, Marsh R, Hays GC (2012) Natal site and offshore swimming influence fitness and long-distance ocean transport in young sea turtles. Mar Biol 159(10):2117–2126
R Core Team (2018) R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing
Ramirez MD, Avens L, Seminoff JA, Goshe LR, Heppell SS (2015) Patterns of loggerhead turtle ontogenetic shifts revealed through isotopic analysis of annual skeletal growth increments. Ecosphere 6(11):1–17
Rees AF, Margaritoulis D, Newman R, Riggall TE, Tsaros P, Zbinden JA, Godley BJ (2013) Ecology of loggerhead marine turtles Caretta caretta in a neritic foraging habitat: movements, sex ratios and growth rates. Mar Biol 160(3):519–529
Rees AF, Alfaro-Shigueto J, Barata PCR, Bjorndal KA, Bolten AB, Bourjea J, Broderick AC, Campbell LM, Cardona L, Carreras C, Casale P (2016) Are we working towards global research priorities for management and conservation of sea turtles? Endang Spec Res 31:337–382
Reich KJ, Bjorndal KA, Del Rio CM (2008) Effects of growth and tissue type on the kinetics of 13C and 15N incorporation in a rapidly growing ectotherm. Oecologia 155(4):651–663
Revelles M, Cardona L, Aguilar A, Fernández G (2007) The diet of pelagic loggerhead sea turtles (Caretta caretta) off the Balearic archipelago (western Mediterranean): relevance of long-line baits. J Mar Biol Assoc UK 87(3):805–813
Rubenstein DR, Hobson KA (2004) From birds to butterflies: animal movement patterns and stable isotopes. Trends Ecol Evol 19(5):256–263
Salmon M, Jones TT, Horch KW (2004) Ontogeny of diving and feeding behavior in juvenile seaturtles: leatherback seaturtles (Dermochelys coriacea L) and green seaturtles (Chelonia mydas L) in the Florida Current. J Herpetol 38(1):36–44
Schofield G, Hobson VJ, Fossette S, Lilley MK, Katselidis KA, Hays GC (2010) Fidelity to foraging sites, consistency of migration routes and habitat modulation of home range by sea turtles. Divers Distrib 16(5):840–853
Schofield G, Dimadi A, Fossette S, Katselidis KA, Koutsoubas D, Lilley MK, Luckman A, Pantis JD, Karagouni AD, Hays GC (2013) Satellite tracking large numbers of individuals to infer population level dispersal and core areas for the protection of an endangered species. Divers Distrib 19(7):834–844
Scott R, Marsh R, Hays GC (2012) A little movement orientated to the geomagnetic field makes a big difference in strong flows. Mar Biol 159(3):481–488
Seney EE (2007) Musick JA (2007) Historical diet analysis of loggerhead sea turtles (Caretta caretta) in Virginia. Copeia 2:478–489
Snape RT, Beton D, Broderick AC, Çiçek BA, Fuller WJ, Özden Ö, Godley BJ (2013) Strand monitoring and anthropological surveys provide insight into marine turtle bycatch in small-scale fisheries of the Eastern Mediterranean. Chelonian Conserv Biol 12(1):44–55
Snover ML, Hohn AA, Crowder LB, Macko SA (2010) Combining stable isotopes and skeletal growth marks to detect habitat shifts in juvenile loggerhead sea turtles Caretta caretta. Endang Spec Res 13(1):25–31
Thomson JA, Heithaus MR, Burkholder DA, Vaudo JJ, Wirsing AJ, Dill LM (2012) Site specialists, diet generalists? Isotopic variation, site fidelity, and foraging by loggerhead turtles in Shark Bay, Western Australia. Mar Ecol Prog Ser 453:213–226
Thomson JA, Whitman ER, Garcia-Rojas MI, Bellgrove A, Ekins M, Hays GC, Heithaus MR (2018) Individual specialization in a migratory grazer reflects long-term diet selectivity on a foraging ground: implications for isotope-based tracking. Oecologia 188(2):429–439
Timpane-Padgham BL, Beechie T, Klinger T (2017) A systematic review of ecological attributes that confer resilience to climate change in environmental restoration. PLoS ONE 12(3):e0173812
Tomás J, Aznar FJ, Raga JA (2001) Feeding ecology of the loggerhead turtle Caretta caretta in the western Mediterranean. J Zool 255(4):525–532
Turner Tomaszewicz CN, Seminoff JA, Peckham SH, Avens L, Kurle CM (2017) Intrapopulation variability in the timing of ontogenetic habitat shifts in sea turtles revealed using δ15N values from bone growth rings. J Anim Ecol 86(3):694–704
Vander Zanden HB, Bjorndal KA, Reich KJ, Bolten AB (2010) Individual specialists in a generalist population: results from a long-term stable isotope series. Biol Let 6(5):711–714
Violle C, Enquist BJ, McGill BJ, Jiang LIN, Albert CH, Hulshof C, Jung V, Messier J (2012) The return of the variance: intraspecific variability in community ecology. Trends Ecol Evol 27(4):244–252
Wallace BP, Avens L, Braun-McNeill J, McClellan CM (2009) The diet composition of immature loggerheads: Insights on trophic niche, growth rates, and fisheries interactions. J Exp Mar Biol Ecol 373(1):50–57
Werner EE, Gilliam JF (1984) The ontogenetic niche and species interactions in size-structured populations. Annu Rev Ecol Syst 15(1):393–425
Wildermann NE, Gredzens C, Avens L, Barrios-Garrido HA, Bell I, Blumenthal J, Bolten AB, McNeill JB, Casale P, Di Domenico M, Domit C (2018) Informing research priorities for immature sea turtles through expert elicitation. Endang Spec Res 37:55–76
Wood SN (2011) Fast stable restricted maximum likelihood and marginal likelihood estimation of semiparametric generalized linear models. JR Stat Soc 73(1):3–36
Zbinden JA, Aebischer A, Margaritoulis D, Arlettaz R (2008) Important areas at sea for adult loggerhead sea turtles in the Mediterranean Sea: satellite tracking corroborates findings from potentially biased sources. Mar Biol 153(5):899–906
Zbinden JA, Bearhop S, Bradshaw P, Gill B, Margaritoulis D, Newton J, Godley BJ (2011) Migratory dichotomy and associated phenotypic variation in marine turtles revealed by satellite tracking and stable isotope analysis. Mar Ecol Prog Ser 421:291–302
Acknowledgements
JCH is supported by a GW4 + Doctoral Training Partnership studentship from the Natural Environment Research Council [NE/L002434/1]. We thank the following for their support: Marine Turtle Conservation Project (MTCP), MAVA foundation, North Cyprus Presidency, seaturtle.org, Society for the Protection of Turtles in North Cyprus (SPOT), and for funding: Apache, BP Egypt, British High Commission in Cyprus and British Residents Society of North Cyprus, Darwin Initiative, Erwin Warth Foundation, Karshiyaka Turtle Watch, Kuzey Kıbrıs Turkcell, MEDASSET, and Natural Environment Research Council (NERC). Capture of turtles in Amvrakikos Gulf was undertaken under permit by the Ministry of Environment. In the NE Adriatic, the study was carried out with support of the Slovenian Research Agency under Grant P1-0386, under the permits Nos. 612-07/97-31/67 and 531-06/1-02-2 of the Ministry of Environmental Protection and Physical Planning of Croatia, and the permits Nos. 354-09-66/00 and 35714-165/01 of the Ministry of the Environment, Spatial Planning and Energy of Slovenia. Work undertaken in North Cyprus was under the permission of the North Cyprus Department for Environmental Protection. For help in collection of turtles, we are thankful to Valter Žiža and to collaborating fishermen in Croatia and Slovenia. We would also like to thank the ARCHELON volunteers in Greece and the numerous Marine Turtle Conservation Project volunteers in North Cyprus for their hardwork during fieldwork. Original artwork used in Fig. 1 is courtesy of Emma Wood. We thank the two anonymous reviewers and the editor, whose inputs have greatly improved the manuscript.
Author information
Authors and Affiliations
Contributions
AB, BG, JS, SW, and WF conceived the project ideas; AB, ALR, BG, BL, DF, DM, JH, NSD, PC, RS, and WF collected the data; JH analysed the data and drafted the manuscript; AB, BG, and JS guided the writing with contributions from all authors. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicting interests.
Ethical approval
All live turtles are either released immediately after sampling or placed under the care of a veterinary surgeon at a rehab centre.
Additional information
Responsible Editor: L. Avens.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Reviewed by undisclosed experts.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Haywood, J.C., Casale, P., Freggi, D. et al. Foraging ecology of Mediterranean juvenile loggerhead turtles: insights from C and N stable isotope ratios. Mar Biol 167, 28 (2020). https://doi.org/10.1007/s00227-020-3647-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-020-3647-5