Abstract
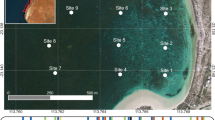
Desmophyllum dianthus is a cosmopolitan species usually found at 1000–2500 m depth in the deep ocean, but in the Patagonian fjords can be found in shallow waters up to 8 m due to deep-water emergence. The purpose of this study was to determine the reproductive biology and seasonality of the scleractinian cup coral D. dianthus from the Chilean fjord region using histological techniques. Corals were collected via SCUBA approximately every 3 months from August 2012 to September 2013 from three sites—Lilihuapi (n = 76) and Punta Huinay (n = 59) in the Comau Fjord; and Morro Gonzalo (n = 44) in the Reñihué Fjord (42.0°S–42.35°S). This study determined that D. dianthus is dioecious, and reproduction is highly seasonal, spawning at the end of austral winter (August) and beginning gamete production in early spring (September). Gametogenesis tracks with known fjord primary productivity and the fjords were coolest and most saline in August, potentially cueing spawning. Owing to the presence of late-stage oocytes in August 2012 and the absence of larvae, we hypothesize that D. dianthus’s mode of reproduction is broadcast spawning. Oogenesis starts in September with previtellogenic oocytes (25–200 μm) that slowly develop into vitellogenic oocytes (200–380 μm) by June. Fecundity is high compared to other deep-sea scleractinians, ranging from 2448 (± 5.13 SE) to 172,328 (± 103.67 SE) potential oocytes per polyp. This research provides the first insight into Desmophyllum dianthus’s reproductive biology and yields an important baseline for continuing work on this benthic habitat builder.






Similar content being viewed by others
References
Addamo AM, Reimer JD, Taviani M, Freiwald A, Machordom A (2012) Desmophyllum dianthus (Esper, 1794) in the Scleractinian phylogeny and its intraspecific diversity. PLoS One 7:e50215
Adkins JF, Henderson GM, Wang SL, O’Shea S, Mokadem F (2004) Growth rates of the deep-sea scleractinia Desmophyllum cristagalli and Enallopsammia rostrata. Earth Planet Sci Lett 227:481–490
Babcock RC, Bull GD, Harrison PL, Heyward AJ, Oliver JK, Wallace CC, Willis BL (1986) Synchronous spawnings of 105 scleractinian coral species on the Great Barrier Reef. Mar Biol 90:379–394
Baillon S, Hamel JF, Mercier A (2011) Comparative study of reproductive synchrony at various scales in deep-sea echinoderms. Deep Sea Res Part 1 Oceanogr Res Pap 58:260–272
Baird AH, Guest JR, Willis BL (2009) Systematic and biogeographical patterns in the reproductive biology of scleractinian corals. Annu Rev Ecol Evol Syst 40:551–571
Bongiorni L, Shafir S, Angel D, Rinkevich B (2003) Survival, growth and gonad development of two hermatypic corals subjected to in situ fish farm enrichment. Mar Ecol Prog Ser 253:137–144
Braga-Henriques A, Porteiro FM, Ribeiro PA, De Matos V, Sampaio Í, Ocaña O, Santos RS (2013) Diversity, distribution and spatial structure of the cold-water coral fauna of the Azores (NE Atlantic). Biogeosci Discuss 10:4009–4036
Brancato MS, Bowlby CE, Hyland J, Intelmann SS, Brenkman K (2007) Observations of deep coral and sponge assemblages in Olympic coast national marine sanctuary, Washington. Cruise Report: NOAA ship McArthur II Cruise AR06-06/07. Marine Sanctuaries Conversation Series
Brooke S, Järnegren J (2013) Reproductive periodicity of the scleractinian coral Lophelia pertusa from the Trondheim Fjord, Norway. Mar Biol 160:139–153
Brooke SD, Young CM (2003) Reproductive ecology of a deep-water scleractinian coral, Oculina varicosa, from the southeast Florida shelf. Cont Shelf Res 23:847–858
Burgess S, Babcock RC (2005) Reproductive Ecology of three reef-forming, deep-sea corals in the New Zealand region. In: Freiwald A, Roberts JM (eds) Cold-water corals and ecosystems. Springer, New York, pp 701–713
Buschmann AH, Cabello F, Young K, Carvajal J, Varela DA, Henríquez L (2009) Salmon aquaculture and coastal ecosystem health in Chile: analysis of regulations, environmental impacts and bioremediation systems. Ocean Coast Manag 52:43–249
Cairns SD (1995) The marine fauna of New Zealand: Scleractinia (Cnidaria, Anthozoa). NZ Oceanogr Inst Mem 103:139–210
Cairns SD (2007) Deep-water corals: an overview with special reference to diversity and distribution of deep-water scleractinian corals. Bull Mar Sci 81:311–322
Eckelbarger KJ, Watling L (1995) Role of phylogenetic constraints in determining reproductive patterns in deep-sea invertebrates. Invertebr Biol 114:256–269
Feehan KA, Waller RG (2015) Notes on reproduction of eight species of Eastern Pacific cold-water octocorals. J Mar Biol Assoc UK 95:691–696
Fillinger L, Richter C (2013) Vertical and horizontal distribution of Desmophyllum dianthus in Comau Fjord, Chile: a cold-water coral thriving at low pH. PeerJ 1:e194
Flint H, Waller RG, Tyler PA (2007) Reproduction in Fungiacyathus marenzelleri from the northeast Pacific Ocean. Mar Biol 151:843–849
Försterra G, Häussermann V (2003) First report on large scleractinian (Cnidaria: Anthozoa) accumulations in cold-temperate shallow water of south Chilean fjords. Zoologische Verhandelingen 345:117–128
Försterra G, Häussermann V, Laudien J, Jantzen C, Sellanes J, Muñoz P (2014) Mass die-off of the cold-water coral Desmophyllum dianthus in the Chilean Patagonian fjord region. Bull Mar Sci 90:895–899
Försterra G, Häussermann V, Laudien J (2017) Animal forests in the Chilean fiord region: Discoveries and perspectives in shallow and deep waters. In: Rossi S (ed) Marine animal forests. p 35. https://doi.org/10.1007/978-3-319-17001-5_3-1
Freiwald A, Fosså JH, Grehan A, Koslow T, Roberts JM (2004) Cold-water coral reefs. UNEP-WCMC Cambridge, Cambridge, p 84
Goffredo S, Arnone S, Zaccanti F (2002) Sexual reproduction in the Mediterranean solitary coral Balanophyllia europaea (Scleractinia, Dendrophylliidae). Mar Ecol Prog Ser 229:83–94
Goffredo S, Gasparini G, Marconi G, Putignano MT, Pazzini C, Zaccanti F (2010) Gonochorism and planula brooding in the Mediterranean endemic orange coral Astroides calycularis (Scleractinia: Dendrophylliidae) morphological aspects of gametogenesis and ontogenesis. Mar Biol Res 6:421–436
Gooday AJ (2002) Biological responses to seasonally varying fluxes of organic matter to the ocean floor: a review. J Oceanogr 58:305–332
Gori A, Ferrier-Pagès C, Hennige SJ, Murray F, Rottier C, Wicks LC, Roberts JM (2016) Physiological response of the cold-water coral Desmophyllum dianthus to thermal stress and ocean acidification. PeerJ 4:e1606
Harrison PL (2011) Sexual reproduction of scleractinian corals. In: Dubinsky Z, Stambler N (eds) Coral reefs: an ecosystem in transition. Springer, Dordrecht, pp 59–85
Harrison PL, Wallace CC (1990) Reproduction, dispersal and recruitment of scleractinian corals. Ecosyst World 25:133–207
Harrison P, Ward S (2001) Elevated levels of nitrogen and phosphorus reduce fertilization success of gametes from scleractinian reef corals. Mar Biol 139:1057–1068
Häussermann V, Förstera G (2009) Marine benthic fauna of Chilean Patagonia, 1st edn. Nature in Focus, Puerto Montt, Chile
Häussermann V, Försterra G, Melzer RR, Meyer R (2013) Gradual changes of benthic biodiversity in Comau fjord, Chilean Patagonia—lateral observations over a decade of taxonomic research. Spixiana 36:161–171
Iriarte JL, González HE, Liu KK, Rivas C, Valenzuela C (2007) Spatial and temporal variability of chlorophyll and primary productivity in surface waters of southern Chile (41.5–43 S). Estuar Coast Shelf Sci 74:471–480
Jantzen C, Laudien J, Sokol S, Försterra G, Häussermann V, Kupprat F, Richter C (2013) In situ short-term growth rates of a cold-water coral. Mar Freshw Res 64:631–641. https://doi.org/10.1071/MF12200
Keller NB (1976) The deep-sea madreporarian corals of the genus Fungiacyathus from the Kurile-Kamchatka, Aleutian Trenches and other regions of the world oceans. Trudy Inst Okeanol 99:31–44
Laudien J, Baumgarten S, Jantzen C, Richter C, Steinmetz R, Häussermann V, Försterra G (2012) Water temperature at time series station Liliguapi, Paso Comau, Patagonia, Chile in 2010. In: Alfred Wegener Institute, Helmholtz Center for Polar and Marine Research, Bremerhaven. https://doi.org/10.1594/pangaea.783296
Laudien J, Jantzen C, Häussermann V, Försterra G (2012) Water temperature at time series station Liliguapi, Paso Comau, Patagonia, Chile in 2011/2012. Alfred Wegener Institute, Helmholtz Center for Polar and Marine Research, Bremerhaven. https://doi.org/10.1594/pangaea.777752
Laudien J, Jantzen C, Häussermann V, Försterra G (2013) Water temperature at time series station Liliguapi, Paso Comau, Patagonia, Chile in 2012/2013. In: Alfred Wegener Institute, Helmholtz Center for Polar and Marine Research, Bremerhaven. https://doi.org/10.1594/pangaea.818388
Laudien J, Häussermann V, Försterra G (2015) Water temperature at time series station Liliguapi, Paso Comau, Patagonia, Chile in 2014/2015. In: Alfred Wegener Institute, Helmholtz Center for Polar and Marine Research, Bremerhaven. https://doi.org/10.1594/pangaea.843773
Lawrence JM, Herrera J (2000) Stress and deviant reproduction in echinoderms. Zool Stud 39:151–171
Mangubhai S, Harrison PL (2008) Asynchronous coral spawning patterns on equatorial reefs in Kenya. Mar Ecol Prog Ser 360:85–96
Mercier A, Hamel JF (2009) Reproductive periodicity and host-specific settlement and growth of a deep-water symbiotic sea anemone. Can J Zool 87:967–980
Mercier A, Sun Z, Hamel JF (2011) Reproductive periodicity, spawning and development of the deep-sea scleractinian coral Flabellum angulare. Mar Biol 158:371–380
Montero P, Daneri G, Tapia F, Iriarte JL, Crawford D (2017a) Diatom blooms and primary production in a channel ecosystem of central Patagonia. Latin Am J Aquat Res 45:999–1016
Montero P, Pérez-Santos I, Daneri G, Gutiérrez MH, Igor G, Seguel R, Purdie A, Crawford DW (2017b) A winter dinoflagellate bloom drives high rates of primary production in a Patagonian fjord ecosystem. Estuar Coast Shelf Sci 199:105–116
Niklitschek EJ, Soto D, Lafon A, Molinet C, Toledo P (2013) Southward expansion of the Chilean salmon industry in the Patagonian fjords: main environmental challenges. Rev Aquac 5:172–195
Pankhurst NW, Van Der Kraak G (1997) Effects of stress on reproduction and growth of fish. Fish stress and health in aquaculture. Cambridge University Press, Cambridge, pp 73–93
Parker NR, Mladenov PV, Grange KR (1997) Reproductive biology of the antipatharian black coral Antipathes fiordensis in Doubtful Sound, Fiordland, New Zealand. Mar Biol 130:11–22
Pires DO, Silva JC, Bastos ND (2014) Reproduction of deep-sea reef-building corals from the southwestern Atlantic. Deep Sea Res Part 2 Top Stud Oceanogr 99:51–63
Rakka M, Orejas C, Sampaio I, Monteiro J, Parra H, Carreiro-Silva M (2017) Reproductive biology of the black coral Antipathella wollastoni (Cnidaria: Antipatharia) in the Azores (NE Atlantic). Deep Sea Res Part 2 Top Stud 145:131–141
Richmond R (1997) Reproduction and recruitment in corals: Critical links to the persistence of reefs. In: Birkeland C (ed) Life and death of coral reefs. Chapman & Hall, London, pp 175–197
Richmond RH, Hunter CL (1990) Reproduction and recruitment of corals: comparisons among the Caribbean, the Tropical Pacific, and the Red Sea. Mar Ecol Prog Ser 60:185–203
Riegl B, Branch GM (1995) Effects of sediment on the energy budgets of four scleractinian (Bourne 1900) and five alcyonacean (Lamouroux 1816) corals. J Exp Mar Biol Ecol 186:259–275
Robert JM, Wheeler AJ, Freiwald A (2006) Reefs of the deep: the biology and geology of cold-water coral ecosystems. Science 312:543–547
Roberts JM (2009) Cold-water corals: the biology and geology of deep-sea coral habitats. Cambridge University Press, Cambridge
Roberts S, Hirshfield M (2004) Deep-sea corals: out of sight, but no longer out of mind. Front Ecol Evol 2:123–130
Rogers CS (1983) Sublethal and lethal effects of sediments applied to common Caribbean reef corals in the field. Mar Pollut Bull 14:378–382
Rogers CS (1990) Responses of coral reefs and reef organisms to sedimentation. Mar Ecol Prog Ser 62:185–202
Rossin AM, Waller RG, Försterra G (2017) Reproduction of the cold-water coral Primnoella chilensis (Philippi, 1894). Cont Shelf Res 144:31–37
Schwabe E, Foersterra G, Haeussermann V, Melzer RR, Schroedl M (2006) Chitons (Mollusca: Polyplacophora) from the southern Chilean Comau Fjord, with reinstatement of Tonicia calbucensis Plate, 1897. Zootaxa 1341:1–27
Stone RP, Shotwell KS (2007) State of deep coral ecosystems in the Alaska Region: Gulf of Alaska, Bering Sea and the Aleutian Islands. In: The state of deep coral ecosystems of the United States. NOAA Technical Memorandum CRCP-3, Silver Spring, Maryland 65-108
Strathmann MF (1987) Reproduction and development of marine invertebrates of the northern Pacific coast: data and methods for the study of eggs, embryos, and larvae. University of Washington Press, Seattle
Tyler PA, Harvey R, Giles LA, Gage JD (1992) Reproductive strategies and diet in deep-sea nuculanid protobranchs (Bivalvia: Nuculoidea) from the Rockall Trough. Mar Biol 114:571–580
Tyler PA, Gage JD, Paterson GJL, Rice AL (1993) Dietary constraints on reproductive periodicity in two sympatric deep-sea astropectinid seastars. Mar Biol 115:267–277
Van Veghel MLJ, Bak RPM (1994) Reproductive characteristics of the polymorphic Caribbean reef-building coral Monastrea annularis. III Reproduction in damaged and regenerating colonies. Mar Ecol Prog Ser 109:229–233
Veron JEN (1995) Corals in space and time: the biogeography and evolution of the Scleractinia. Cornell University Press, Ithaca
Waller RG (2005) Deep-water Scleractinia (Cnidaria: Anthozoa): current knowledge of reproductive processes. Cold-water corals and ecosystems. Springer, Berlin, pp 691–700
Waller RG, Feehan KA (2013) Reproductive ecology of a polar deep-sea scleractinian, Fungiacyathus marenzelleri (Vaughan, 1906). Deep Sea Res Part 2 Top Stud Oceanogr 92:201–206
Waller RG, Tyler PA (2005) The reproductive biology of two deep-water, reef-building scleractinians from the NE Atlantic Ocean. Coral Reefs 24:514–522
Waller R, Tyler P, Gage J (2002) Reproductive ecology of the deep-sea scleractinian coral Fungiacyathus marenzelleri (Vaughan, 1906) in the northeast Atlantic Ocean. Coral Reefs 21:325–331
Waller RG, Tyler PA, Gage JD (2005) Sexual reproduction of three deep water Caryophyllia (Anthozoa: Scleractinia) species from the NE Atlantic Ocean. Coral Reefs 24(4):594–602
Waller RG, Tyler PA, Smith C (2008) Fecundity and embryo development of three Antarctic deep-water scleractinians: Flabellum thouarsii, F. curvatum and F. impensum. Deep Sea Res Part 2 Top Stud Oceanogr 55:2527–2553
Waller RG, Stone RP, Johnstone J, Mondragon J (2014) Sexual reproduction and seasonality of the Alaskan Red Tree Coral, Primnoa pacifica. PLoS One 9:e90893
Wisshak M, Freiwald A, Lundälv T, Gektidis M (2005) The physical niche of the bathyal Lophelia pertusa in a non-bathyal setting: environmental controls and palaeoecological implications. Cold-water corals and ecosystems. Springer, Berlin, pp 979–1001
Young CM (2003) Reproduction, development and life-history traits. Ecosyst World , pp 381–426
Zakai D, Levy O, Chadwick-Furman NE (2000) Experimental fragmentation reduces sexual reproductive output by the reef-building coral Pocillopora damicornis. Coral Reefs 19:185–188
Zibrowius H (1980) Les Scléractiniaires de la Méditerranée et de l’Atlantique nord-oriental. Mémoires de l’Institut océanographique, Monaco, pp 118–119
Acknowledgements
We would like to thank the Huinay Scientific Field Station for being the base camp for the field portion of this study. Our deepest thanks and appreciation for Dr. Laura Grange, Chris Riguad and all divers and scientists who collected samples for this study. In addition, we would like to thank Dr. Kevin Ecklebarger, Dr. Robert Steneck and Dr. Damian Brady for their advisement on this project. We thank University of Maine undergraduate Maggie Halfman and graduate students Ashley Rossin and Elise Hartill for their help with processing. Lastly, we thank the two anonymous reviewers for their helpful comments and edits of this paper. This research would not have been possible without the support of National Geographic (GEFNE26-11) and the National Science Foundation (OCE-1219554), and funding for field work was partially provided through Fondecyt project number 1150843 and 1161699. This is publication number 141 of Huinay Scientific Field Station.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
This work was funded by National Geographic and the National Science Foundation (USA), and the authors declare there are no conflicts of interest.
Ethical approval
All applicable international, national and/or institutional guidelines for sampling, care and experimental use of organisms were followed and all necessary approvals have been obtained.
Additional information
Responsible Editor: by D. Gochfeld.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Reviewed by Undisclosed experts.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Feehan, K.A., Waller, R.G. & Häussermann, V. Highly seasonal reproduction in deep-water emergent Desmophyllum dianthus (Scleractinia: Caryophylliidae) from the Northern Patagonian Fjords. Mar Biol 166, 52 (2019). https://doi.org/10.1007/s00227-019-3495-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-019-3495-3