Abstract
Purpose
Bone health and body composition share several common mechanisms like oxidative stress and inflammation. Anthocyanins have antioxidant and anti-inflammatory properties. We have reported that anthocyanins are associated with better body composition in children, but the associations with bone health have not been elucidated. We aimed to explore the association of anthocyanins with bone mineral content (BMC) and bone mineral density (BMD) at multiple sites in children.
Methods
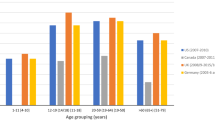
In this cross-sectional study, 452 Chinese children aged 6–9 years were recruited. A validated 79-item food frequency questionnaire was used to collect dietary information. BMC and BMD at multiple sites (whole body; whole body excluding head, WBEH; limbs; arms; legs) were measured by dual-energy X-ray.
Results
Higher dietary intake of total anthocyanidins (per one standard deviation increase) was associated with a 1.28–13.6 g (1.31-1.60%, compared to median) higher BMC at all sites and a 3.61–6.96 mg (0.65–0.90%) higher BMD at the whole body, WBEH, and arm sites after controlling for a number of possible covariates. The results were similar and more pronounced for cyanidin, but not for delphinidin and peonidin. Higher dietary intake of cyanidin (per one standard deviation increase) was associated with a 1.33–15.4 g (1.48–1.68%) higher BMC at all sites and a 4.15–7.77 mg (0.66–1.00%) higher BMD at all sites except the legs. No statistically significant associations with BMC or BMD were found for dietary intake of delphinidin and peonidin.
Conclusions
Higher dietary intake of total anthocyanidins and cyanidins were associated with higher BMC and BMD in Chinese children.
Similar content being viewed by others
References
Tella SH, Gallagher JC (2014) Prevention and treatment of postmenopausal osteoporosis. J Steroid Biochem Mol Biol 142:155–170. https://doi.org/10.1016/j.jsbmb.2013.09.008
Li H, Xiao Z, Quarles LD, Li W (2021) Osteoporosis: mechanism, molecular target and current status on drug development. Curr Med Chem 28:1489–1507. https://doi.org/10.2174/0929867327666200330142432
Chen GD, Dong XW, Zhu YY, Tian HY, He J, Chen YM (2016) Adherence to the Mediterranean diet is associated with a higher BMD in middle-aged and elderly Chinese. Sci Rep 6:25662. https://doi.org/10.1038/srep25662
Qiu R, Cao WT, Tian HY, He J, Chen GD, Chen YM (2017) Greater intake of fruit and vegetables is associated with greater bone mineral density and lower osteoporosis risk in middle-aged and elderly adults. PLoS ONE 12:e0168906. https://doi.org/10.1371/journal.pone.0168906
Panchal SK, John OD, Mathai ML, Brown L (2022) Anthocyanins in chronic diseases: the power of purple. Nutrients 14:2161. https://doi.org/10.3390/nu14102161
Hair R, Sakaki JR, Chun OK (2021) Anthocyanins, microbiome and health benefits in aging. Molecules 26:537. https://doi.org/10.3390/molecules26030537
Speer H, D’Cunha NM, Alexopoulos NI, McKune AJ, Naumovski N (2020) Anthocyanins and human health-A focus on oxidative stress. Inflamm Dis Antioxid (Basel) 9:366. https://doi.org/10.3390/antiox9050366
Chen L, Hu B, Wang X, Chen Y, Zhou B (2022) Functional role of cyanidin-3-O-glucoside in osteogenesis: a pilot study based on RNA-seq analysis. Front Nutr 9:995643. https://doi.org/10.3389/fnut.2022.995643
Ren Z, Raut NA, Lawal TO, Patel SR, Lee SM, Mahady GB (2021) Peonidin-3-O-glucoside and cyanidin increase osteoblast differentiation and reduce RANKL-induced bone resorption in transgenic medaka. Phytother Res 35:6255–6269. https://doi.org/10.1002/ptr.7271
Hu B, Chen L, Chen Y, Zhang Z, Wang X, Zhou B (2021) Cyanidin-3-glucoside regulates osteoblast differentiation via the ERK1/2 signaling pathway. ACS Omega 6:4759–4766. https://doi.org/10.1021/acsomega.0c05603
Imangali N, Phan QT, Mahady G, Winkler C (2021) The dietary anthocyanin delphinidin prevents bone resorption by inhibiting Rankl-induced differentiation of osteoclasts in a medaka (Oryzias latipes) model of osteoporosis. J Fish Biol 98:1018–1030. https://doi.org/10.1111/jfb.14317
Sakaki J, Melough M, Lee SG, Kalinowski J, Koo SI, Lee SK, Chun OK (2018) Blackcurrant supplementation improves trabecular bone mass in young but not aged mice. Nutrients 10:1671. https://doi.org/10.3390/nu10111671
Cheng J, Zhou L, Liu Q, Tickner J, Tan Z, Li X, Liu M, Lin X, Wang T, Pavlos NJ, Zhao J, Xu J (2018) Cyanidin chloride inhibits ovariectomy-induced osteoporosis by suppressing RANKL-mediated osteoclastogenesis and associated signaling pathways. J Cell Physiol 233:2502–2512. https://doi.org/10.1002/jcp.26126
Dou C, Li J, Kang F, Cao Z, Yang X, Jiang H, Yang B, Xiang J, Xu J, Dong S (2016) Dual effect of cyanidin on RANKL-induced differentiation and fusion of osteoclasts. J Cell Physiol 231:558–567. https://doi.org/10.1002/jcp.24916
Park KH, Gu DR, So HS, Kim KJ, Lee SH (2015) Dual role of cyanidin-3-glucoside on the differentiation of bone cells. J Dent Res 94:1676–1683. https://doi.org/10.1177/0022034515604620
Moriwaki S, Suzuki K, Muramatsu M, Nomura A, Inoue F, Into T, Yoshiko Y, Niida S (2014) Delphinidin, one of the major anthocyanidins, prevents bone loss through the inhibition of excessive osteoclastogenesis in osteoporosis model mice. PLoS ONE 9:e97177. https://doi.org/10.1371/journal.pone.0097177
Lee SG, Kim B, Yang Y, Pham TX, Park YK, Manatou J, Koo SI, Chun OK, Lee JY (2014) Berry anthocyanins suppress the expression and secretion of proinflammatory mediators in macrophages by inhibiting nuclear translocation of NF-κB independent of NRF2-mediated mechanism. J Nutr Biochem 25:404–411. https://doi.org/10.1016/j.jnutbio.2013.12.001
Zheng X, Mun S, Lee SG, Vance TM, Hubert P, Koo SI, Lee SK, Chun OK (2016) Anthocyanin-rich blackcurrant extract attenuates ovariectomy-induced bone loss in mice. J Med Food 19:390–397. https://doi.org/10.1089/jmf.2015.0148
Hardcastle AC, Aucott L, Reid DM, Macdonald HM (2011) Associations between dietary flavonoid intakes and bone health in a Scottish population. J Bone Miner Res 26:941–947. https://doi.org/10.1002/jbmr.285
Welch A, MacGregor A, Jennings A, Fairweather-Tait S, Spector T, Cassidy A (2012) Habitual flavonoid intakes are positively associated with bone mineral density in women. J Bone Miner Res 27:1872–1878. https://doi.org/10.1002/jbmr.1649
Zhang ZQ, He LP, Liu YH, Liu J, Su YX, Chen YM (2014) Association between dietary intake of flavonoid and bone mineral density in middle aged and elderly Chinese women and men. Osteoporos Int 25:2417–2425. https://doi.org/10.1007/s00198-014-2763-9
Chen G, Li Y, Liang S, Xiao J, Duan X, Zhou Y, Zeng Y, Sun F, Shrestha S, Zhang Z (2021) Associations of dietary anthocyanidins intake with body composition in Chinese children: a cross-sectional study. Food Nutr Res 65:4428. https://doi.org/10.29219/fnr.v65.4428
Chen G, Yan H, Hao Y, Shrestha S, Wang J, Li Y, Wei Y, Pan J, Zhang Z (2019) Comparison of various anthropometric indices in predicting abdominal obesity in Chinese children: a cross-sectional study. BMC Pediatr 19:127. https://doi.org/10.1186/s12887-019-1501-z
Zhang CX, Ho SC (2009) Validity and reproducibility of a food frequency questionnaire among Chinese women in Guangdong province. Asia Pac J Clin Nutr 18:240–250
Yang YX, Wang GY, Pan XW (2009) China food composition table. Peking University Medical Press, Beijing
Yang Y (2018) China Food Composition Table. Peking University Medical Press, Beijing
Bouchard C, Tremblay A, Leblanc C, Lortie G, Savard R, Thériault G (1983) A method to assess energy expenditure in children and adults. Am J Clin Nutr 37:461–467. https://doi.org/10.1093/ajcn/37.3.461
Dodier T, Anderson KL, Bothwell J, Hermann J, Lucas EA, Smith BJ (2021) U.S. Montmorency tart cherry juice decreases bone resorption in women aged 65–80 years. Nutrients 13:544. https://doi.org/10.3390/nu13020544
Gabel L, Macdonald HM, McKay HA (2017) Sex differences and growth-related adaptations in bone microarchitecture, geometry, density, and strength from childhood to early adulthood: a mixed longitudinal HR-pQCT Study. J Bone Miner Res 32:250–263. https://doi.org/10.1002/jbmr.2982
Hasselstrøm H, Karlsson KM, Hansen SE, Grønfeldt V, Froberg K, Andersen LB (2006) Sex differences in bone size and bone mineral density exist before puberty. The Copenhagen School Child Intervention Study (CoSCIS). Calcif Tissue Int 79:7–14. https://doi.org/10.1007/s00223-006-0012-8
Iantomasi T, Romagnoli C, Palmini G, Donati S, Falsetti I, Miglietta F, Aurilia C, Marini F, Giusti F, Brandi ML (2023) Oxidative stress and inflammation in osteoporosis: molecular mechanisms involved and the relationship with microRNAs. Int J Mol Sci 24:3772. https://doi.org/10.3390/ijms24043772
Wakefield CB, Lee VR, Johnston D, Boroumand P, Pillon NJ, Sayedyahossein S, O’Donnell BL, Tang J, Sanchez-Pupo RE, Barr KJ, Gros R, Flynn L, Borradaile NM, Klip A, Beier F, Penuela S (2022) Pannexin 3 deletion reduces fat accumulation and inflammation in a sex-specific manner. Int J Obes 46:726–738. https://doi.org/10.1038/s41366-021-01037-4
Noutsios GT, Thorenoor N, Zhang X, Phelps DS, Umstead TM, Durrani F, Floros J (2019) Major effect of oxidative stress on the male, but not female, SP-A1 type II Cell miRNome. Front Immunol 10:1514. https://doi.org/10.3389/fimmu.2019.01514
Noutsios GT, Thorenoor N, Zhang X, Phelps DS, Umstead TM, Durrani F, Floros J (2017) SP-A2 contributes to miRNA-mediated sex differences in response to oxidative stress: pro-inflammatory, anti-apoptotic, and anti-oxidant pathways are involved. Biol Sex Differ 8:37. https://doi.org/10.1186/s13293-017-0158-2
Bloor ID, Symonds ME (2014) Sexual dimorphism in white and brown adipose tissue with obesity and inflammation. Horm Behav 66:95–103. https://doi.org/10.1016/j.yhbeh.2014.02.007
Wang XX, Lin KN, Xu WC, Chen H (2022) The causal relationship between abdominal obesity and lower bone mineral density: A two-sample mendelian randomization study. Front Genet 13:970223. https://doi.org/10.3389/fgene.2022.970223
Acknowledgements
The authors would like to thank all research members involved in the data collection of the study.
Funding
This work was supported by National Natural Science Foundation of China (No.81502798, ZZQ), Natural Science Foundation of Guangdong Province, China (Grant No. 2015A030310399, ZZQ), the Maternal and Children Nutrition and Care Fund of Biostime (Grant No. BINCMYF15006, ZZQ), National Natural Science Foundation of China (Grant No. 82103855, G.D.C.,), Basic and Applied Basic Research Foundation of Guangdong Province (Grant No. 2019A1515110163, G.D.C.,) and the Foundation of Bureau of Science and Technology of Foshan City (Grant No. 2220001004104, G.D.C.,). The funding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
Author information
Authors and Affiliations
Contributions
GDC and SJL: analyzed the data and wrote the paper. LH, HRY, and YUW: were parts of the data collection team; QZW and ZQZ: revised the manuscript; ZQZ: designed the project, supervised the study and revised the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
Geng-dong Chen, Shu-jun Liang, Lan Huang, Hao-ran Yu, Yu-lin Wu, Qin-zhi Wei and Zhe-qing Zhang have no competing interests to declare that are relevant to the content of this article.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study was approved by the Ethics Committee of the School of Public Health, Sun Yat-sen University (No. 201549).
Human and Animal Rights and Informed Consent
The Study was conducted in accordance with the tenets of the Declaration of Helsinki and was approved by the Ethics Committee of the School of Public Health, Sun Yat-sen University (No. 201549).
Informed Consent
Well informed consent was obtained from each subject included in the study through his/her parents or legal guardians.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, Gd., Liang, Sj., Huang, L. et al. Associations of Dietary Anthocyanidins Intake with Bone Health in Children: A Cross-Sectional Study. Calcif Tissue Int 113, 393–402 (2023). https://doi.org/10.1007/s00223-023-01128-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-023-01128-6