Abstract
Hereditary hemochromatosis (HHC) is characterized by excessive intestinal iron absorption resulting in a pathological increase of iron levels. Parenchyma damage may be a consequence of iron deposition in affected organs (e.g., liver, pancreas, gonads) as well as bones and joints, leading to osteoporosis with increased fracture risk and arthropathy. Up to date, it is not known whether HHC can also be considered as a risk factor for osteonecrosis. Likewise, the underlying skeletal changes are unknown regarding, e.g., microstructural properties of bone. We aimed to study the spectrum of skeletal complications in HHC and the possible underlying microarchitectural changes. Therefore, we retrospectively analyzed all patients with HHC (n = 10) presenting in our outpatient clinic for bone diseases. In addition to dual-energy X-ray absorptiometry (DXA), high-resolution peripheral quantitative computed tomography (HR-pQCT) was performed and bone turnover markers, 25-OH-D3, ferritin and transferrin saturation were measured. Cortical volumetric bone mineral density (Ct.BMD) and cortical thickness (Ct.Th) were reduced, whereas trabecular microstructure (Tb.Th) and volumetric bone mineral density (Tb.BMD) were preserved compared to age- and gender-adjusted reference values from the literature. Interestingly, the occurrence of bone complications was age dependent; while younger patients presented with osteonecroses or transient bone marrow edema, patients older than 65 years presented with fractures. Our study provides first insights into altered bone microarchitecture in HHC and sheds new light on the occurrence of osteonecrosis. If available, HR-pQCT is a useful complement to fracture risk assessment and to determine microstructural deterioration and volumetric bone mineralization deficits.
Similar content being viewed by others
Introduction
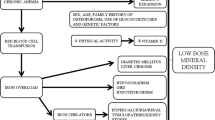
Hereditary hemochromatosis (HHC) is a systemic disorder characterized by iron accumulation in the human body which may lead to symptomatic organ involvement with iron deposition and destructive processes not only in the liver, but also in the pancreas, heart, hormone glands, or joints. Five different mutations are known to date that may lead to HHC with different penetrance. The most common mutation in populations of European descent is located in the HFE gene, the so-called HFE-related HHC [1], in which the C282Y mutation is found in most cases [2]. As mutations in the hepcidin gene (HAMP) cause juvenile hemochromatosis in humans [3] and lack of hepcidin gene expression in upstream stimulatory factor 2 (USF2) knockout mice leads to iron overload [4], hepcidin is considered as a key regulator in iron metabolism [5, 6].
It is well known that not only patients with chronic liver diseases with cholestasis such as primary biliary cholangitis (PBC) or primary sclerosing cholangitis [7, 8], but also those without cholestasis including hemochromatosis have an increased risk of osteoporosis. At least 25 percent of HHC patients suffer from osteoporosis [9]. Rising hepatic iron concentrations are associated with low femoral bone mineral density (BMD) in male patients with HFE-related HHC [10]. It is assumed that the iron overload itself leads to osteoporosis rather than a specific genetic mutation [11], which is supported by the association of osteoporosis and the amount of iron removed by phlebotomy in HHC patients [12]. Iron infusions (ferric carboxymaltose) are reported to elevate fibroblast growth factor 23 (FGF23) secretion of osteocytes leading to renal phosphate wasting, calcitriol deficiency, and secondary hyperparathyroidism [13]. Whether an increased oral iron uptake as in HHC has the same effect on FGF23 secretion and consecutive osteoporosis or osteomalacia is still unclear. Moreover, excess iron is reported to inhibit osteoblastic cell proliferation, differentiation, and primary mineralization in vitro [14], possibly inducing low bone formation [15]. Additionally, a secondary impairment of mineralization by direct inhibition of iron on hydroxyapatite crystal growth has been described in vitro [16]. In animal studies with iron-overloaded mice, increased reactive oxygen species and dose-dependent tissue iron content were determined, leading to impaired bone microarchitecture, i.e., reduced cortical and trabecular thickness, as well as increased non-mineralized matrix [17]. Treatment with antioxidants prevented trabecular, but not cortical bone changes in these iron-overloaded mice. Taken together, several animal and human studies provide further evidence of a bone affection in hemochromatosis. Whether this is leading to an increased risk of fracture has hardly been investigated specifically to date. Single case reports found osteoporosis-associated fractures in two HHC patients [18, 19]. In a cross-sectional survey, significantly more HHC patients had been diagnosed with osteoporosis than age- and gender-matched controls [20]. Severe iron overload, reflected by serum ferritin levels at diagnosis above 1000 µg/l, was associated with wrist or vertebral fractures. The survey also revealed a significantly higher prevalence of painful joints, osteoarthritis, and the presence of hip replacement in HHC. Osteoarthritis is a common symptom of HHC with a high percentage of patients undergoing joint replacement [21]. Beyond that, painful joints could not only be a sign of osteoarthritis, but also of primary osteonecrosis, a complication that has so far been detected in only very few cases [22,23,24].
The aim of this retrospective study was to determine whether there are microarchitectural changes in bone that explain the occurrence of fractures and whether osteonecrosis is another relevant complication of HHC.
Materials and Methods
Study Group and Detailed Skeletal Assessment
We included all patients with HHC who had presented in our outpatient clinic for bone diseases between 2013 and 2018 in this retrospective study. HHC was diagnosed by an internal specialist based on a clinical examination, elevated iron metabolism parameters, HFE gene examination, as well as further diagnostic procedures such as liver biopsy or non-invasive superconducting quantum interference device (SQUID) liver biomagnetometry [25] and MRI in some cases. At the time of the presentation in our outpatient clinic, nine of ten patients had already been diagnosed with HHC, but in one patient, the suspicion of hemochromatosis was raised due to a laboratory co-determination of ferritin/transferrin saturation and was confirmed afterwards. A total of 10 patients with HHC underwent routine bone assessment due to the presence of skeletal complications or risk factors. Dual-energy X-ray absorptiometry (Lunar iDXA, GE Healthcare, Madison, WI, USA) at the lumbar spine and proximal femur was performed. The lowest T- and Z-scores at the lumbar spine (mean of at least two vertebrae) and proximal femur (neck or total) were used for further analysis. Laboratory assessments included serum calcium, phosphate, creatinine, 25-hydroxyvitamin D (25-OH-D3), alkaline phosphatase (ALP), bone-specific alkaline phosphatase (bAP), osteocalcin, parathyroid hormone (PTH), transferrin saturation, ferritin, and urinary desoxypyridinoline (DPD crosslinks). The medical history of skeletal complications had been assessed in all patients. Regular therapeutic phlebotomy was reported by nine patients. In seven patients, a genetic mutation in the HFE gene was detected, while in three patients no genetic analysis was conducted as this was not desired by the patients. None of the patients received specific anti-osteoporotic treatment (e.g., bisphosphonates, denosumab, teriparatide) at the time of the initial bone assessment except one female patient who was previously treated with i.v. ibandronate a few times due to the occurrence of multiple osteonecroses. The diagnosis of osteonecrosis was based on radiographs and MRI; in the case of femoral head necrosis, the diagnosis was based on the ARCO (Association Research Circulation Osseous) classification system [26]. ARCO stages I and II represent an irreversible early stage of osteonecrosis with positive MRI only (stage I) or both positive radiography and MRI (stage II), whereas ARCO stages III and IV are characterized by positive radiography or CT showing subchondral infraction (stage III) or secondary osteoarthritis (stage IV). Common causes of osteonecrosis including corticosteroids, alcohol, trauma, hemopathies, and vasculitis were excluded [27]. All procedures performed in this study were in accordance with the Declaration of Helsinki and with the guidelines of the local ethics committee (WF-038/19).
HR-pQCT
In addition, data from high-resolution peripheral quantitative computed tomography (HR-pQCT) at the non-dominant distal radius and contralateral distal tibia was available for all HHC patients. In cases of a reported previous fracture, the opposite extremity was scanned. Default in vivo settings for HR-pQCT assessment with 60 kVp, 1000 µA, 100 ms integration time and an isotropic voxel size of 82 µm (XtremeCT, SCANCO Medical AG, Brüttisellen, Switzerland) [28] and the manufacturer’s standard protocol was used to measure bone microstructure and volumetric bone mineral density. According to the ASBMR guidelines for nomenclature [29], microstructure parameters including cortical thickness (Ct.Th, mm), trabecular thickness (Tb.Th, mm), trabecular number (Tb.N, 1/mm), and bone volume to total volume ratio (BV/TV) were calculated. Microstructure parameters were supplemented by volumetric bone mineral density (BMD, mgHA/cm3) measurements, i.e., calculation of total (Tt.BMD), cortical (Ct.BMD), and trabecular BMD (Tb.BMD). To obtain reliable and accurate values, daily quality control with a calibration phantom provided by the manufacturer was performed. The patients’ HR-pQCT parameters were compared with age- and gender-adjusted reference values provided by a previous study using the same scanner and protocol [30].
Statistical Analysis
Normal distribution of data was tested with the Kolmogorow–Smirnow test. As the data was not normally distributed, relationships between ferritin, transferrin saturation, bone laboratory parameters, DXA values, and HR-pQCT-parameters were tested by Spearman’s rank correlation analysis. Bone microstructure and volumetric bone mineral density parameters of the patients were plotted on age-dependent percentile curves provided by Burt et al. for visual comparison with reference values [30]. For quantitative comparison purposes, the percentage of the patients' HR-pQCT-parameters compared to age- and gender-adjusted reference values from the literature were calculated. Group differences within the patient cohort were analyzed using Mann–Whitney U tests. Statistical differences were regarded as significant for p < 0.05. If not stated otherwise, we report the median and interquartile range (IQR) of the data. All statistical analyses were conducted using SPSS 22.0 software (IBM, Armonk, NY, USA).
Results
Patient Characteristics
Our study cohort consisted of n = 4 females and n = 6 males with a total median age of 58.5 years. Table 1 shows demographic and disease-specific data of all patients as well as the localization of skeletal complications including irreversible osteonecrosis (n = 5), classified as ARCO stage I or higher in the case of femoral head necrosis, diffuse bone marrow edema (n = 3), and fragility fractures (n = 3). Representative examples of different bone manifestations in HHC are shown in Fig. 1a–d. Case 3 was particularly severely affected by HHC. At diagnosis, ferritin levels between 700–900 µg/l were observed with extremely severe iron overload of the liver, i.e., the iron overload was clearly underestimated by the ferritin levels in this case. Clinically, the female patient with a homozygous C282Y mutation developed multiple osteonecroses of both the loaded lower limb and the unloaded upper limb. Hip joints and knee joints had to be replaced bilaterally within a few years and she also showed erosive arthritis of the carpal (Fig. 1d) and metacarpal bones of the hand. Other causes of osteonecrosis or destructive osteoarthritis were excluded by the rheumatologist. In this case, an unknown genetic effect leading to the unusual iron storage and possibly also to the unusual bone findings seems likely.
Representative examples of different bone affections in HHC. a Osteonecrosis in MRI T1- or PD-weighted, b vertebral fracture in lateral vertebral fracture assessment (VFA) using DXA, c bone marrow edema in PD-weighted MRI sequence, d erosive osteoarthritis in HR-pQCT of the carpal bones (left) and T1-weighted MR images (right)
In most patients, laboratory parameters indicating bone mineralization and turnover were within the reference range (Table 2). Median ferritin levels and transferrin saturation were elevated, but the variance was large depending on the severity of the disease and the frequency of therapeutic phlebotomy. All patients except one received regular phlebotomies at the time when they presented themselves in our specialized outpatient clinic for bone diseases. Four patients presented shortly after the beginning of the therapy with phlebotomies (Case 1, 2, 9, and 10), i.e., serum ferritin and transferrin saturation were still elevated and not yet in the target range. In long-term treated patients (Case 3, 4, 6, 7, and 8) target serum ferritin < 50 µg/l was not always achievable, e.g., if patients showed hemoglobin levels lower than 12 g/dl.
Urinary DPD crosslinks were elevated in three men and one woman indicating activated bone resorption in these patients. There were no significant correlations between ferritin or transferrin saturation and osteological laboratory parameters.
Median DXA T-Score was normal at the lumbar spine and in the range of moderate osteopenia at the proximal femur according to the World Health Organization (WHO) criteria (Fig. 2a). Only in two cases, DXA T-scores were within the range of osteoporosis according to WHO.
Bone mineral density and bone microarchitecture. a Individual DXA T-Scores of the lumbar spine (LS, mean of at least two vertebrae) as well as right (R) and left (L) hip (lowest of femoral neck or total hip) for all patients and the resulting median T-Score (dash). In some patients, DXA was not possible at all sites due to implants or degenerative lumbar spine disease. These values are not depicted. b Representative high-resolution peripheral quantitative computed tomography (HR-pQCT) image showing the cortical thinning at the distal radius and distal tibia. (c, d) Cortical thickness (Ct.Th), (e, f) cortical bone mineral density (Ct.BMD), g, h trabecular thickness (Tb.Th), and (i, j) trabecular bone mineral density (Tb.BMD) for male and female patients measured at the distal radius and distal tibia. Age- and gender-dependent reference range of HR-pQCT parameters displayed as percentile curves (10th, 25th, 50th, 75th, and 90th) provided by Burt et al. [30]
Bone Microarchitecture and Volumetric Density
A representative example of the bone microarchitecture is shown in Fig. 2b. Compared to age- and gender-adjusted reference values provided by Burt et al., the patients’ median Ct.Th was markedly reduced (radius: − 26.1%; tibia: − 32.3%; Fig. 2c, d). Ct.BMD (radius: − 15.2%; tibia: − 13.3%; Fig. 2e, f) and Tt.BMD (radius: − 11.4%; tibia: − 16.7%) were moderately reduced, whereas Tb.Th (radius: − 6.6%; tibia: − 9.2%; Fig. 2g, h) and Tb.BMD (radius: − 5.0%; tibia: − 3.7%; Fig. 2i, j) were relatively preserved.
Structural and volumetric deficits were similar between male and female patients (Table 3).
To investigate a relationship with iron metabolism, ferritin and transferrin saturation were correlated with HR-pQCT parameters, but no significant correlations were found.
Subgroup Analysis: Osteonecrosis vs. Fragility Fracture
In the second step, we analyzed HHC patients with osteonecrosis (n = 5) and fragility fractures (n = 3) separately. Interestingly, HHC patients presented with osteonecrosis up to 61 years of age, while patients with fractures were older than 70 years. Both groups differed significantly in age (55 vs. 75 years; p < 0.05), but not in BMI, laboratory bone metabolism parameters, DXA, and HR-pQCT values (all p > 0.05). Neither median transferrin saturation (88.5% vs. 82%) nor serum ferritin (474 ng/ml vs. 51 ng/ml) differed significantly between patients with osteonecrosis and patients with fragility fractures (both p > 0.05). Patients with fractures showed non-significantly lower cortical thickness at the radius (0.54 vs. 0.71 mm) and the tibia (0.52 vs. 1.0 mm) compared to the osteonecrosis group (Table 4). When comparing both groups after adjusting for age and gender by calculating the relative percentage of age- and gender-adjusted reference values, Ct.Th and Ct.BMD were still lower in the fracture group. Although these differences failed to reach statistical significance, different bone manifestations do not seem to be based entirely on the age effect.
Discussion
Osteoporosis is a known complication of HHC, but studies concerning further skeletal manifestations including fractures or osteonecrosis are poorly documented and microarchitectural bone properties are unknown. We therefore retrospectively analyzed ten HHC patients regarding altered structural and volumetric bone data assessed by HR-pQCT. In addition, DXA osteodensitometry, bone laboratory analyses, and the occurrence of skeletal events were also evaluated after the patients’ initial presentation.
Key Findings
HHC can affect the bone in various ways. Available data is limited and mainly concentrated on studies on osteoporosis [10, 11] or osteoarthritis [12, 31, 32]. We showed that fractures as well as osteonecroses can be relevant complications of HHC patients presenting in an outpatient clinic for bone diseases. Osteonecroses occurred in five of ten HHC patients in our study cohort and in two patients even multifocally. In addition to previously published (case)studies on bone affection in HHC in humans and mice, our results provide further insights into HHC-related impairment of bone microarchitecture. We found a pronounced cortical bone loss with mineralization deficits reflected by reduced cortical thickness and cortical bone mineral density.
Fractures and Osteonecrosis in Hemochromatosis
Fractures have been described to be a presenting feature of hemochromatosis [18, 19] and a large survey revealed a borderline significant prevalence of wrist and vertebral fractures in HHC patients compared to healthy controls [20]. In patients with secondary iron overload such as thalassemia syndromes a high fracture prevalence is described [33, 34]. On the other hand, evidence for HHC-related osteonecrosis is rare, but described in very few cases [22,23,24]. To our knowledge, the description of five further cases of osteonecrosis in HHC represents the largest cohort described in the literature to date. Interestingly, we found an age-dependent bone manifestation pattern in our HHC patients. On an average, HHC patients with fragility fractures were 20 years older than HHC patients with osteonecrosis, but bone structure and metabolism parameters did not differ significantly. One could speculate that osteonecrosis is mainly related to disturbed trabecular bone architecture, whereas cortical thickness predominantly defines fracture risk. Accordingly, patients with fragility fractures showed age- and gender-independent reduced cortical thickness at the radius and the tibia as measured by HR-pQCT. These results may not have been significant due to the small group size. Similar to HHC, there are other diseases with a predominant cortical bone loss. For example, a pronounced cortical bone loss with relatively preserved peripheral trabecular microstructure in HR-pQCT was also observed in type 2 diabetics [35] with cortical porosity-related fractures. This may explain the occurrence of fractures in our subgroup of HHC patients despite a median T-Score of − 1.9 since DXA mainly measures bone mineral density of trabecular bone, at least at the spine. An altered bone microarchitecture was also found in PBC patients, while their DXA values were not significantly lower compared to controls [7].
Pathophysiological Explanatory Approaches
In animal studies with different iron overload mouse models, micro-CT analysis revealed trabecular [36] as well as cortical bone loss with an increase of non-mineralized matrix and a decrease of carbonate-to-mineral ratio in FTIRI analysis indicating osteomalacia and increased bone turnover [17]. Our HHC patients also showed a reduced mineralization of the cortical bone, i.e., low Ct and BMD values. Several aspects can be considered as possible explanations for bone loss and hypomineralization in hemochromatosis in general. Both reduced bone formation and increased bone resorption appear to play a role in influencing the bone [37, 38]. A reduced bone formation by a direct [14] or a radical-mediated effect of iron on osteoblasts is discussed [17] and a secondary impairment of mineralization by the inhibition of hydroxyapatite crystal growth by iron is described in vitro [16]. Other in vitro studies on human osteoblasts suggested that the ferroxidase activity of ferritin that inhibits the mineralization and downregulates the alkaline phosphate expression and activity [39], both, could be explanations for low cortical BMD. Iron selectively inhibits differentiation of multipotent mesenchymal stem cells to osteoblasts [40]. This is in line with studies on HHC patients, a negative impact of hepatic iron concentrations on BMD, [10] and an association of osteoporosis with the severity of iron overload at diagnosis (reflected by serum ferritin levels or iron removed to reach depletion), but transferrin saturation was not determined [11, 20]. Compared to our HHC patient cohort, median serum ferritin levels were only slightly elevated and did not correlate with BMD or bone microarchitectural changes. One reason for this might be that the available iron metabolism parameters were not from the time of the initial diagnosis and that the progression of HHC was individually different. Phlebotomies were performed regularly in the majority of patients, which explains the partly normal iron metabolism parameters, but a relation to iron removed to reach depletion could not be analyzed due to incomplete records. According to the latest findings, iron-induced FGF23 secretion could also play a role in the development of osteoporosis and osteomalacia. After ferric carboxymaltose administration, elevated FGF23 levels were found in patients with iron deficiency anemia leading to renal phosphate loss and hypophosphatemia [13]. However, it is questionable whether the same mechanism also applies to increased oral iron uptake in HHC and if this explains reduced cortical BMD in our patients as an indication of osteomalacia. While phosphate levels were normal in all included patients, FGF23 was not routinely determined. However, this remains an interesting subject for future studies.
On the other hand, there are in vitro studies showing that iron excess facilitates osteoclast differentiation and influences osteoclastic activity [37]. Altered osteoclastogenesis due to transferrin receptor 1 (Tfr1)-mediated iron uptake [41] and the expression of TRAP regulated by iron [42] both may explain bone loss in HHC-affected humans. Additional evidence comes from iron-overloaded C57/BL6 mice by iron dextran injections since the bone tissue contained increased number of osteoclasts [17]. Increased bone resorption caused by low levels of testosterone could also explain our results because hypogonadism may appear secondary to HHC [1, 43]. In our cohort, we cannot rule out that male patients were hypogonadal, since we did not measure current testosterone levels at the time of presentation.
Furthermore, other metal cations (e.g., zinc, manganese, aluminum) are reported to interfere with iron metabolism [44]. In patients with HFE-related hemochromatosis, hepatic zinc concentration was increased fivefold compared to healthy controls suggesting increased intestinal absorption of zinc and iron in hemochromatosis [45]. Additionally, altered expression of iron transporters influences manganese transport possibly modifying manganese-induced neurotoxicity [46]. Aluminum is also known to inhibit skeletal mineralization as it can substitute calcium in mineralized bone matrix [47, 48]. Beside positive iron staining in the bone matrix of patients with HHC, aluminum can be found as linear deposits in some patients as well. These studies show that metabolism of other metal cations is linked to iron metabolism, i.e., iron-induced changes within the bone matrix in HHC may be further aggravated by other metal cations or that other metal cations can also cause a change in the composition of bone matrix and the associated risk of osteonecrosis, bone marrow edema, or fractures.
Limitations of the Study
There are several limitations of this study that need to be acknowledged. The prevalence of fractures and osteonecroses may be biased as we are a specialized outpatient clinic for bone diseases (i.e., patients without skeletal complications were not seen at our department). Therefore, no statement regarding the prevalence of skeletal complications can be made based on this study. Age is a major risk factor for the development of osteoporosis and the associated fragility fractures, which is also reflected in our cohort with increasing age. However, an age-independent factor can also be assumed, influencing the bone microstructure and thus the occurrence of osteonecroses or fractures that could only be determined in its tendency due to the small cohort size. Other explanations for the structural and volumetric bone changes such as 25-OH-D3 deficiency or hyperparathyroidism are rather unlikely as these parameters were normal in most of our patients. As elevated transferrin saturation under maintenance therapy is reported to promote joint pain in HHC [49] and especially case 3 suffering from multiple osteonecroses and bone marrow edema in the presence of high transferrin saturation under long-term treatment, it is conceivable that joint pain in HHC is partly caused by osteonecrosis and that persistent high levels of transferrin saturation could be a risk factor for the development of osteonecrosis. However, due to the small cohort size, different severity of the liver disease between HHC patients and the varying blood sample collection (shortly after phlebotomy initiation or during long-term maintenance therapy), general conclusions regarding effects of persisting elevated transferrin saturation or impaired bone microarchitecture in HHC are not possible.
Conclusion
Taken together, our study makes an important contribution to the knowledge of bone involvement in HHC and shows that osteonecrosis may also occur as another bone manifestation alongside osteoporosis and fractures, especially in patients younger than 60 years. The solitary measurement of areal BMD obtained by DXA may have its limitations in fracture risk assessment in patients with HHC, since we found bone microstructural changes, i.e., bone loss and mineralization deficits, primarily in cortical bone which cannot be detected sufficiently by DXA. If available, HR-pQCT measurement can be a useful complement to fracture risk assessment in HHC. Just like other known forms of secondary osteonecrosis (e.g., induced by high-dose corticosteroid therapy, alcohol abuse, chemotherapy, radiation, systemic lupus erythematosus, or sickle cell anemia) HHC should also be considered by the treating physician by looking for typical symptoms and performing laboratory analyses of serum ferritin, transferrin saturation, and if necessary genetic testing. Further studies with larger numbers of patients with HHC are required to assess the bone microarchitecture and prevalence of bone manifestations with varying disease severity before, under, and after treatment.
References
Pietrangelo A (2004) Hereditary hemochromatosis—a new look at an old disease. N Engl J Med 350:2383–2397
Feder JN, Gnirke A, Thomas W, Tsuchihashi Z, Ruddy DA, Basava A, Dormishian F, Domingo R Jr, Ellis MC, Fullan A, Hinton LM, Jones NL, Kimmel BE, Kronmal GS, Lauer P, Lee VK, Loeb DB, Mapa FA, McClelland E, Meyer NC, Mintier GA, Moeller N, Moore T, Morikang E, Prass CE, Quintana L, Starnes SM, Schatzman RC, Brunke KJ, Drayna DT, Risch NJ, Bacon BR, Wolff RK (1996) A novel MHC class I-like gene is mutated in patients with hereditary haemochromatosis. Nat Genet 13:399–408
Roetto A, Papanikolaou G, Politou M, Alberti F, Girelli D, Christakis J, Loukopoulos D, Camaschella C (2003) Mutant antimicrobial peptide hepcidin is associated with severe juvenile hemochromatosis. Nat Genet 33:21–22
Pigeon C, Ilyin G, Courselaud B, Leroyer P, Turlin B, Brissot P, Loreal O (2001) A new mouse liver-specific gene, encoding a protein homologous to human antimicrobial peptide hepcidin, is overexpressed during iron overload. J Biol Chem 276:7811–7819
Fleming RE, Sly WS (2001) Hepcidin: a putative iron-regulatory hormone relevant to hereditary hemochromatosis and the anemia of chronic disease. Proc Natl Acad Sci USA 98:8160–8162
Ganz T (2003) Hepcidin, a key regulator of iron metabolism and mediator of anemia of inflammation. Blood 102:783–788
Schmidt T, Schmidt C, Schmidt FN, Butscheidt S, Mussawy H, Hubert J, Hawellek T, Oehler N, Barvencik F, Lohse AW, Schinke T, Schramm C, Amling M, Rolvien T (2018) Disease duration and stage influence bone microstructure in patients with primary biliary cholangitis. J Bone Miner Res 33:1011–1019
Schmidt T, Schwinge D, Rolvien T, Jeschke A, Schmidt C, Neven M, Butscheidt S, Kriz M, Kunzmann L, Mussawy H, Hubert J, Hawellek T, Ruther W, Oheim R, Barvencik F, Lohse AW, Schramm C, Schinke T, Amling M (2019) Th17 cell frequency is associated with low bone mass in primary sclerosing cholangitis. J Hepatol 70:941–953
Guanabens N, Pares A (2018) Osteoporosis in chronic liver disease. Liver Int 38:776–785
Guggenbuhl P, Deugnier Y, Boisdet JF, Rolland Y, Perdriger A, Pawlotsky Y, Chales G (2005) Bone mineral density in men with genetic hemochromatosis and HFE gene mutation. Osteoporos Int 16:1809–1814
Valenti L, Varenna M, Fracanzani AL, Rossi V, Fargion S, Sinigaglia L (2009) Association between iron overload and osteoporosis in patients with hereditary hemochromatosis. Osteoporos Int 20:549–555
Sinigaglia L, Fargion S, Fracanzani AL, Binelli L, Battafarano N, Varenna M, Piperno A, Fiorelli G (1997) Bone and joint involvement in genetic hemochromatosis: role of cirrhosis and iron overload. J Rheumatol 24:1809–1813
Wolf M, Chertow GM, Macdougall IC, Kaper R, Krop J, Strauss W (2018) Randomized trial of intravenous iron-induced hypophosphatemia. JCI Insight 3:e124486
Yamasaki K, Hagiwara H (2009) Excess iron inhibits osteoblast metabolism. Toxicol Lett 191:211–215
Doyard M, Chappard D, Leroyer P, Roth MP, Loreal O, Guggenbuhl P (2016) Decreased bone formation explains osteoporosis in a genetic mouse model of hemochromatosiss. PLoS ONE 11:e0148292
Guggenbuhl P, Filmon R, Mabilleau G, Basle MF, Chappard D (2008) Iron inhibits hydroxyapatite crystal growth in vitro. Metabolism 57:903–910
Tsay J, Yang Z, Ross FP, Cunningham-Rundles S, Lin H, Coleman R, Mayer-Kuckuk P, Doty SB, Grady RW, Giardina PJ, Boskey AL, Vogiatzi MG (2010) Bone loss caused by iron overload in a murine model: importance of oxidative stress. Blood 116:2582–2589
Eyres KS, McCloskey EV, Fern ED, Rogers S, Beneton M, Aaron JE, Kanis JA (1992) Osteoporotic fractures: an unusual presentation of haemochromatosis. Bone 13:431–433
Duquenne M, Rohmer V, Legrand E, Chappard D, Wion Barbot N, Basle MF, Audran M, Bigorgne JC (1996) Spontaneous multiple vertebral fractures revealed primary haemochromatosis. Osteoporos Int 6:338–340
Richette P, Ottaviani S, Vicaut E, Bardin T (2010) Musculoskeletal complications of hereditary hemochromatosis: a case-control study. J Rheumatol 37:2145–2150
Sahinbegovic E, Dallos T, Aigner E, Axmann R, Manger B, Englbrecht M, Schoniger-Hekele M, Karonitsch T, Stamm T, Farkas M, Karger T, Stolzel U, Keysser G, Datz C, Schett G, Zwerina J (2010) Musculoskeletal disease burden of hereditary hemochromatosis. Arthritis Rheum 62:3792–3798
Montgomery KD, Williams JR, Sculco TP, DiCarlo E (1998) Clinical and pathologic findings in hemochromatosis hip arthropathy. Clin Orthop Relat Res 347:179–187
Rollot F, Wechsler B, du Boutin le TH, De Gennes C, Amoura Z, Hachulla E, Piette JC (2005) Hemochromatosis and femoral head aseptic osteonecrosis: a nonfortuitous association? J Rheumatol 32:376–378
Jaffres R (1966) Bilateral aseptic osteonecrosis of the hip in a patient with hemochromatosis. Rev Rhum Mal Osteoartic 33:269–272
Nielsen P, Fischer R, Engelhardt R, Tondury P, Gabbe EE, Janka GE (1995) Liver iron stores in patients with secondary haemosiderosis under iron chelation therapy with deferoxamine or deferiprone. Br J Haematol 91:827–833
Gardeniers JWM (1991) ARCO committee on terminology and staging (report from the Nijmegen meeting). ARCO News Lett 3:153–159
Assouline-Dayan Y, Chang C, Greenspan A, Shoenfeld Y, Gershwin ME (2002) Pathogenesis and natural history of osteonecrosis. Semin Arthritis Rheum 32:94–124
Milovanovic P, Adamu U, Simon MJ, Rolvien T, Djuric M, Amling M, Busse B (2015) Age- and sex-specific bone structure patterns portend bone fragility in radii and tibiae in relation to osteodensitometry: a high-resolution peripheral quantitative computed tomography study in 385 individuals. J Gerontol A Biol Sci Med Sci 70:1269–1275
Bouxsein ML, Boyd SK, Christiansen BA, Guldberg RE, Jepsen KJ, Muller R (2010) Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J Bone Miner Res 25:1468–1486
Burt LA, Liang Z, Sajobi TT, Hanley DA, Boyd SK (2016) Sex- and site-specific normative data curves for HR-pQCT. J Bone Miner Res 31:2041–2047
Carroll GJ, Breidahl WH, Bulsara MK, Olynyk JK (2011) Hereditary hemochromatosis is characterized by a clinically definable arthropathy that correlates with iron load. Arthritis Rheum 63:286–294
Elstob A, Ejindu V, Heron CW, Kiely PDW (2018) MRI ankle and subtalar characteristics in haemochromatosis arthropathy: a case-control study. Clin Radiol 73(323):e321–e328
Vogiatzi MG, Macklin EA, Fung EB, Vichinsky E, Olivieri N, Kwiatkowski J, Cohen A, Neufeld E, Giardina PJ (2006) Prevalence of fractures among the Thalassemia syndromes in North America. Bone 38:571–575
Vogiatzi MG, Macklin EA, Fung EB, Cheung AM, Vichinsky E, Olivieri N, Kirby M, Kwiatkowski JL, Cunningham M, Holm IA, Lane J, Schneider R, Fleisher M, Grady RW, Peterson CC, Giardina PJ, Thalassemia Clinical Research N (2009) Bone disease in thalassemia: a frequent and still unresolved problem. J Bone Miner Res 24:543–557
Burghardt AJ, Issever AS, Schwartz AV, Davis KA, Masharani U, Majumdar S, Link TM (2010) High-resolution peripheral quantitative computed tomographic imaging of cortical and trabecular bone microarchitecture in patients with type 2 diabetes mellitus. J Clin Endocrinol Metab 95:5045–5055
Simao M, Camacho A, Ostertag A, Cohen-Solal M, Pinto IJ, Porto G, Hang Korng E, Cancela ML (2018) Iron-enriched diet contributes to early onset of osteoporotic phenotype in a mouse model of hereditary hemochromatosis. PLoS ONE 13:e0207441
Jeney V (2017) Clinical impact and cellular mechanisms of iron overload-associated bone loss. Front Pharmacol 8:77
Balogh E, Paragh G, Jeney V (2018) Influence of iron on bone homeostasis. Pharmaceuticals (Basel) 11:107
Zarjou A, Jeney V, Arosio P, Poli M, Zavaczki E, Balla G, Balla J (2010) Ferritin ferroxidase activity: a potent inhibitor of osteogenesis. J Bone Miner Res 25:164–172
Balogh E, Tolnai E, Nagy B Jr, Nagy B, Balla G, Balla J, Jeney V (2016) Iron overload inhibits osteogenic commitment and differentiation of mesenchymal stem cells via the induction of ferritin. Biochim Biophys Acta 1862:1640–1649
Ishii KA, Fumoto T, Iwai K, Takeshita S, Ito M, Shimohata N, Aburatani H, Taketani S, Lelliott CJ, Vidal-Puig A, Ikeda K (2009) Coordination of PGC-1beta and iron uptake in mitochondrial biogenesis and osteoclast activation. Nat Med 15:259–266
Alcantara O, Reddy SV, Roodman GD, Boldt DH (1994) Transcriptional regulation of the tartrate-resistant acid phosphatase (TRAP) gene by iron. Biochem J 298(Pt 2):421–425
Diamond T, Stiel D, Posen S (1989) Osteoporosis in hemochromatosis: iron excess, gonadal deficiency, or other factors? Ann Intern Med 110:430–436
Loreal O, Cavey T, Bardou-Jacquet E, Guggenbuhl P, Ropert M, Brissot P (2014) Iron, hepcidin, and the metal connection. Front Pharmacol 5:128
Adams PC, Bradley C, Frei JV (1991) Hepatic zinc in hemochromatosis. Clin Invest Med 14:16–20
Ye Q, Park JE, Gugnani K, Betharia S, Pino-Figueroa A, Kim J (2017) Influence of iron metabolism on manganese transport and toxicity. Metallomics 9:1028–1046
Chappard D, Mabilleau G, Moukoko D, Henric N, Steiger V, Le Nay P, Frin JM, De Bodman C (2015) Aluminum and iron can be deposited in the calcified matrix of bone exostoses. J Inorg Biochem 152:174–179
Chappard D, Bizot P, Mabilleau G, Hubert L (2016) Aluminum and bone: Review of new clinical circumstances associated with Al(3+) deposition in the calcified matrix of bone. Morphologie 100:95–105
Bardou-Jacquet E, Laine F, Guggenbuhl P, Morcet J, Jezequel C, Guyader D, Moirand R, Deugnier Y (2017) Worse outcomes of patients with HFE hemochromatosis with persistent increases in transferrin saturation during maintenance therapy. Clin Gastroenterol Hepatol 15:1620–1627
Acknowledgments
Open Access funding provided by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
Conceptualization: NMJ, FB; Methodology: NMJ, FB; Formal analysis and investigation: NMJ, TR, TS, HM, PN, RO, MA, and FB; Writing — original draft preparation: NMJ, TR, TS, PN, and FB; Wrtiting — review & editing: NMJ, TR, TS, HM, PN, RO, MA, and FB; Funding acquisition: n/a; Resources: PN, MA; Supervision: PN, FB; Software: SPSS 22 (IBM Corp., Armonk, New York) and Microsoft Power Point & Excel 2016 (Microsoft, Redmond, Washington).
Corresponding author
Ethics declarations
Conflict of interest
Nico Maximilian Jandl, Tim Rolvien, Tobias Schmidt, Haider Mussawy, Peter Nielsen, Ralf Oheim, Michael Amling, and Florian Barvencik declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
All procedures performed in this study were in accordance with the Declaration of Helsinki and approved by the local ethics committee (WF-038/19).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jandl, N.M., Rolvien, T., Schmidt, T. et al. Impaired Bone Microarchitecture in Patients with Hereditary Hemochromatosis and Skeletal Complications. Calcif Tissue Int 106, 465–475 (2020). https://doi.org/10.1007/s00223-020-00658-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-020-00658-7