Abstract
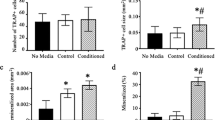
In response to mechanical loading skeletal muscle produces numerous growth factors and cytokines that enter the circulation. We hypothesized that myotubes produce soluble factors that affect osteoclast formation and aimed to identify which osteoclastogenesis-modulating factors are differentially produced by mechanically stimulated myotubes. C2C12 myotubes were subjected to mechanical loading by cyclic strain for 1 h, and postincubated with or without cyclic strain for 24 h. The effect of cyclic strain on gene expression in myotubes was determined by PCR. Conditioned medium (CM) was collected from cultures of unloaded and loaded myotubes and from MLO-Y4 osteocytes. CM was added to mouse bone marrow cells containing osteoclast precursors, and after 6 days osteoclasts were counted. Compared to unconditioned medium, CM from unloaded osteocytes increased osteoclast formation, while CM from unloaded myotubes decreased osteoclast formation. Cyclic strain strongly enhanced IL-6 expression in myotubes. CM from cyclically strained myotubes increased osteoclast formation compared to CM from unloaded myotubes, but this effect did not occur in the presence of an IL-6 antibody. In conclusion, mechanically loaded myotubes secrete soluble factors, among others IL-6, which affect osteoclast formation. These results suggest that muscle could potentially affect bone homeostasis in vivo via production of growth factors and/or cytokines.




Similar content being viewed by others
Abbreviations
- CM:
-
conditioned medium
References
Ruf KM, Johnson NK, Clifford T, Smith KM (2008) Risk factors, prevention, and treatment of corticosteroid-induced osteoporosis in adults. Orthopedics 31:768–772
Walsh MC, Hunter GR, Livingstone MB (2006) Sarcopenia in premenopausal and postmenopausal women with osteopenia, osteoporosis and normal bone mineral density. Osteoporos Int 17:61–67
Armbrecht G, Belavy DL, Gast U, Bongrazio M, Touby F, Beller G, Roth HJ, Perschel FH, Rittweger J, Felsenberg D (2010) Resistive vibration exercise attenuates bone and muscle atrophy in 56 days of bed rest: biochemical markers of bone metabolism. Osteoporos Int 21:597–607
Huijing PA, Jaspers RT (2005) Adaptation of muscle size and myofascial force transmission: a review and some new experimental results. Scand J Med Sci Sports 15:349–380
Juffer P, Jaspers RT, Lips P, Bakker AD, Klein-Nulend J (2012) Expression of muscle anabolic and metabolic factors in mechanically loaded MLO-Y4 osteocytes. Am J Physiol Endocrinol Metab 302:E389–E395
van Wessel T, de Haan A, van der Laarse WJ, Jaspers RT (2010) The muscle fiber type-fiber size paradox: hypertrophy or oxidative metabolism? Eur J Appl Physiol 110:665–694
Rittweger J, Gerrits K, Altenburg T, Reeves N, Maganaris CN, de Haan A (2006) Bone adaptation to altered loading after spinal cord injury: a study of bone and muscle strength. J Musculoskelet Neuronal Interact 6:269–276
Nowlan NC, Bourdon C, Dumas G, Tajbakhsh S, Prendergast PJ, Murphy P (2010) Developing bones are differentially affected by compromised skeletal muscle formation. Bone 46:1275–1285
Hamrick MW, Shi X, Zhang W, Pennington C, Thakore H, Haque M, Kang B, Isales CM, Fulzele S, Wenger KH (2007) Loss of myostatin (GDF8) function increases osteogenic differentiation of bone marrow–derived mesenchymal stem cells but the osteogenic effect is ablated with unloading. Bone 40:1544–1553
Deckers MM, Karperien M, van der Bent C, Yamashita T, Papapoulos SE, Lowik CW (2000) Expression of vascular endothelial growth factors and their receptors during osteoblast differentiation. Endocrinology 141:1667–1674
Deng M, Zhang B, Wang K, Liu F, Xiao H, Zhao J, Liu P, Li Y, Lin F, Wang Y (2010) Mechano growth factor E peptide promotes osteoblasts proliferation and bone-defect healing in rabbits. Int Orthop 35:8
Li SH, Guo DZ, Li B, Yin HB, Li JK, Xiang JM, Deng GZ (2009) The stimulatory effect of insulin-like growth factor-1 on the proliferation, differentiation, and mineralisation of osteoblastic cells from Holstein cattle. Vet J 179:430–436
Hossain M, Irwin R, Baumann MJ, McCabe LR (2005) Hepatocyte growth factor (HGF) adsorption kinetics and enhancement of osteoblast differentiation on hydroxyapatite surfaces. Biomaterials 26:2595–2602
Karasik D, Kiel DP (2010) Evidence for pleiotropic factors in genetics of the musculoskeletal system. Bone 46:1226–1237
Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T, Elliott R, Colombero A, Elliott G, Scully S, Hsu H, Sullivan J, Hawkins N, Davy E, Capparelli C, Eli A, Qian YX, Kaufman S, Sarosi I, Shalhoub V, Senaldi G, Guo J, Delaney J, Boyle WJ (1998) Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell 93:165–176
Bakker AD, Silva VC, Krishnan R, Bacabac RG, Blaauboer ME, Lin YC, Marcantonio RA, Cirelli JA, Klein-Nulend J (2009) Tumor necrosis factor alpha and interleukin-1beta modulate calcium and nitric oxide signaling in mechanically stimulated osteocytes. Arthritis Rheum 60:3336–3345
Kanaji A, Caicedo MS, Virdi AS, Sumner DR, Hallab NJ, Sena K (2009) Co–Cr–Mo alloy particles induce tumor necrosis factor alpha production in MLO-Y4 osteocytes: a role for osteocytes in particle-induced inflammation. Bone 45:528–533
Kim JH, Jin HM, Kim K, Song I, Youn BU, Matsuo K, Kim N (2009) The mechanism of osteoclast differentiation induced by IL-1. J Immunol 183:1862–1870
Rifas L, Weitzmann MN (2009) A novel T cell cytokine, secreted osteoclastogenic factor of activated T cells, induces osteoclast formation in a RANKL-independent manner. Arthritis Rheum 60:3324–3335
Hemingway F, Taylor R, Knowles HJ, Athanasou NA (2011) RANKL-independent human osteoclast formation with APRIL, BAFF, NGF, IGF I and IGF II. Bone 48:938–944
Tan SD, de Vries TJ, Kuijpers-Jagtman AM, Semeins CM, Everts V, Klein-Nulend J (2007) Osteocytes subjected to fluid flow inhibit osteoclast formation and bone resorption. Bone 41:745–751
Kulkarni RN, Bakker AD, Everts V, Klein-Nulend J (2012) Mechanical loading prevents the stimulating effect of IL-1beta on osteocyte-modulated osteoclastogenesis. Biochem Biophys Res Commun 420:11–16
Steensberg A, Keller C, Starkie RL, Osada T, Febbraio MA, Pedersen BK (2002) IL-6 and TNF-alpha expression in, and release from, contracting human skeletal muscle. Am J Physiol Endocrinol Metab 283:E1272–E1278
Goldspink G (2005) Mechanical signals, IGF-I gene splicing, and muscle adaptation. Physiology (Bethesda) 20:232–238
Yaffe D, Saxel O (1977) Serial passaging and differentiation of myogenic cells isolated from dystrophic mouse muscle. Nature 270:725–727
Burkholder TJ, Lieber RL (2001) Sarcomere length operating range of vertebrate muscles during movement. J Exp Biol 204:1529–1536
van der Krogt MM, Doorenbosch CA, Harlaar J (2007) Muscle length and lengthening velocity in voluntary crouch gait. Gait Posture 26:532–538
Tester NJ, Barbeau H, Howland DR, Cantrell A, Behrman AL (2012) Arm and leg coordination during treadmill walking in individuals with motor incomplete spinal cord injury: a preliminary study. Gait Posture 36:49–55
Soltow QA, Lira VA, Betters JL, Long JH, Sellman JE, Zeanah EH, Criswell DS (2010) Nitric oxide regulates stretch-induced proliferation in C2C12 myoblasts. J Muscle Res Cell Motil 31:215–225
Hatfaludy S, Shansky J, Vandenburgh HH (1989) Metabolic alterations induced in cultured skeletal muscle by stretch–relaxation activity. Am J Physiol Cell Physiol 256:C175–C181
Kulkarni RN, Bakker AD, Everts V, Klein-Nulend J (2010) Inhibition of osteoclastogenesis by mechanically loaded osteocytes: involvement of MEPE. Calcif Tissue Int 87:461–468
de Vries TJ, Schoenmaker T, Beertsen W, van der Neut R, Everts V (2005) Effect of CD44 deficiency on in vitro and in vivo osteoclast formation. J Cell Biochem 94:954–966
Pedersen BK (2011) Muscles and their myokines. J Exp Biol 214:337–346
Banfi G, Lombardi G, Colombini A, Lippi G (2010) Bone metabolism markers in sports medicine. Sports Med 40:697–714
Hamrick MW (2011) A role for myokines in muscle–bone interactions. Exerc Sport Sci Rev 39:43–47
Cheung WY, Simmons CA, You L (2012) Osteocyte apoptosis regulates osteoclast precursor adhesion via osteocytic IL-6 secretion and endothelial ICAM-1 expression. Bone 50:104–110
Dai SM, Nishioka K, Yudoh K (2004) Interleukin (IL) 18 stimulates osteoclast formation through synovial T cells in rheumatoid arthritis: comparison with IL1 beta and tumour necrosis factor alpha. Ann Rheum Dis 63:1379–1386
Evans KE, Fox SW (2007) Interleukin-10 inhibits osteoclastogenesis by reducing NFATc1 expression and preventing its translocation to the nucleus. BMC Cell Biol 8:4
Park JS, Jung YO, Oh HJ, Park SJ, Heo YJ, Kang CM, Kwok SK, Ju JH, Park KS, Cho ML, Sung YC, Park SH, Kim HY (2012) Interleukin-27 suppresses osteoclastogenesis via induction of interferon-gamma. Immunology 137:326–335
Thirunavukkarasu K, Miles RR, Halladay DL, Yang X, Galvin RJ, Chandrasekhar S, Martin TJ, Onyia JE (2001) Stimulation of osteoprotegerin (OPG) gene expression by transforming growth factor-beta (TGF-beta). Mapping of the OPG promoter region that mediates TGF-beta effects. J Biol Chem 276:36241–36250
Boyce BF, Xing L (2008) Functions of RANKL/RANK/OPG in bone modeling and remodeling. Arch Biochem Biophys 473:139–146
Henningsen J, Pedersen BK, Kratchmarova I (2011) Quantitative analysis of the secretion of the MCP family of chemokines by muscle cells. Mol Biosyst 7:311–321
Pedersen L, Pilegaard H, Hansen J, Brandt C, Adser H, Hidalgo J, Olesen J, Pedersen B, Hojman P (2011) Exercise-induced liver chemokine CXCL-1 expression is linked to muscle-derived interleukin-6 expression. J Physiol 589:1409–1420
Sims NA, Walsh NC (2010) GP130 cytokines and bone remodelling in health and disease. BMB Rep 43:513–523
Poulton IJ, McGregor NE, Pompolo S, Walker EC, Sims NA (2012) Contrasting roles of leukemia inhibitory factor in murine bone development and remodeling involve region-specific changes in vascularization. J Bone Miner Res 27:586–595
Miyaura C, Kusano K, Masuzawa T, Chaki O, Onoe Y, Aoyagi M, Sasaki T, Tamura T, Koishihara Y, Ohsugi Y et al (1995) Endogenous bone-resorbing factors in estrogen deficiency: cooperative effects of IL-1 and IL-6. J Bone Miner Res 10:1365–1373
Rufo A, Del Fattore A, Capulli M, Carvello F, De Pasquale L, Ferrari S, Pierroz D, Morandi L, De Simone M, Rucci N, Bertini E, Bianchi ML, De Benedetti F, Teti A (2011) Mechanisms inducing low bone density in Duchenne muscular dystrophy in mice and humans. J Bone Miner Res 26:1891–1903
Yang X, Ricciardi BF, Hernandez-Soria A, Shi Y, Pleshko Camacho N, Bostrom MP (2007) Callus mineralization and maturation are delayed during fracture healing in interleukin-6 knockout mice. Bone 41:928–936
Liu XH, Kirschenbaum A, Yao S, Levine AC (2006) Interactive effect of interleukin-6 and prostaglandin E2 on osteoclastogenesis via the OPG/RANKL/RANK system. Ann NY Acad Sci 1068:225–233
Erices A, Conget P, Rojas C, Minguell JJ (2002) gp130 activation by soluble interleukin-6 receptor/interleukin-6 enhances osteoblastic differentiation of human bone marrow-derived mesenchymal stem cells. Exp Cell Res 280:24–32
Fischer CP (2006) Interleukin-6 in acute exercise and training: what is the biological relevance? Exerc Immunol Rev 12:6–33
Kaufman H, Reznick A, Stein H, Barak M, Maor G (2008) The biological basis of the bone–muscle inter-relationship in the algorithm of fracture healing. Orthopedics 31:751
Acknowledgments
The authors thank D. C. Jansen and J. Vermeer, for the isolation of mouse bone marrow cells, and K. Hermes and G. Sowidjojo, for their technical assistance. This work was supported by a grant from the MOVE Research Institute Amsterdam of the VU University Amsterdam, The Netherlands
Author information
Authors and Affiliations
Corresponding author
Additional information
The authors have stated that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Juffer, P., Jaspers, R.T., Klein-Nulend, J. et al. Mechanically Loaded Myotubes Affect Osteoclast Formation. Calcif Tissue Int 94, 319–326 (2014). https://doi.org/10.1007/s00223-013-9813-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-013-9813-8