Abstract
Our purpose was to characterize the risks of osteoporosis-related subtrochanteric fractures in bisphosphonate-naive individuals. Baseline characteristics of patients enrolled in the HORIZON-Recurrent Fracture Trial with a study-qualifying hip fracture were examined, comparing those who sustained incident subtrochanteric fractures with those sustaining other hip fractures. Subjects were bisphosphonate-naive or had a bisphosphonate washout period of 6–24 months and subsequently received an annual infusion of zoledronic acid 5 mg or placebo after low-trauma hip-fracture repair. In total, 2,127 men and women were included. Of the qualifying hip fractures, 5.2% were subtrochanteric, 54.8% femoral neck, 33.0% intertrochanteric, and 7.1% other (generally complex fractures of mixed type). Significant baseline (pre-hip fracture) differences were seen between index hip-fracture types, with the percentage of patients with extreme mobility problems being twofold higher in patients with index subtrochanteric fracture (9.9%) compared to other patients. The distribution of hip-fracture types was similar between the treatment groups at baseline. No patients with index subtrochanteric fractures and six patients with other qualifying hip fractures reported prior bisphosphonate use. Only one further subtrochanteric fracture occurred in each treatment group over an average 2-year patient follow-up. Subtrochanteric fractures are not uncommon in bisphosphonate-naive patients. Extreme difficulties with mobility may be a unique risk factor predisposing to development of incident subtrochanteric fractures rather than other types of hip fracture. In patients with recent hip fracture who received zoledronic acid therapy, the incidence of new subtrochanteric fractures was too small to draw any meaningful conclusions.
Similar content being viewed by others
Recent publications have questioned the safety of bisphosphonates in the treatment of osteoporosis [1–6]. In particular, concern has been raised regarding the development of atypical subtrochanteric fractures in alendronate-treated individuals potentially related to marked suppression of bone turnover [2–4]. Atypical fractures are fractures that occur in diaphyseal and subtrochanteric sites after minimal trauma, such as a fall from standing height or lower. Thickened cortices differentiate these atypical fractures from the thin cortices seen with typical osteoporotic fractures. Subtrochanteric fractures, however, are known to occur in patients suffering from osteoporosis who have never been treated with bisphosphonates. The question then arises whether incident subtrochanteric fractures in bisphosphonate-treated individuals are a consequence of the underlying condition or due to therapy. Little is known about the epidemiology of these osteoporosis-related subtrochanteric fractures in older adults, and such information may be helpful in untangling emerging data about atypical, bisphosphonate-related subtrochanteric fractures.
Patients with a low-trauma hip fracture have one of the highest risks of subsequent fracture, including a high risk of hip-fracture recurrence [7, 8]. In order to better understand the epidemiology and outcomes of subtrochanteric fractures in osteoporotic patients who were bisphosphonate-naive, we examined the baseline characteristics from the HORIZON-Recurrent Fracture Trial (RFT) to determine the risks for incident osteoporosis-related subtrochanteric fractures.
Methods
Design Overview
This was a post-hoc analysis of the HORIZON-RFT, a randomized, placebo-controlled, double-blind study, which recruited subjects aged ≥50 years who had undergone surgical repair of a low-trauma hip fracture in the preceding 90 days [5].
Setting and Participants
The HORIZON-RFT evaluated the safety and efficacy of a once-yearly bisphosphonate, zoledronic acid, in preventing new clinical fractures in a total of 2,127 patients with recent low-trauma hip-fracture repair. Patients were unwilling or unable to take an oral bisphosphonate. Prior use of bisphosphonates or parathyroid hormone was allowed, with a washout period determined by the drug and the duration of its use. The washout periods for any oral bisphosphonate were 2 years (if used for more than 48 weeks), 1 year (if used for 8–48 weeks), and 6 months (if used for 2–8 weeks). This post-hoc analysis focused on subjects with subtrochanteric hip fractures as their qualifying (index) hip fracture for entry into the study, prior to any study drug administration. Index fracture site (subtrochanteric, femoral neck/subcapital, intertrochanteric, or other) was recorded by the investigator at the time of enrollment, but index hip-fracture radiographs were not collected.
Informed consent was obtained from subjects, and investigations were approved by an institutional human research committee. The study was conducted according to the ethical principles of the 1989 Declaration of Helsinki and local applicable laws and regulations.
Randomization and Interventions
Patients were assigned at random to receive an intravenous infusion of zoledronic acid 5 mg (Novartis Pharma, Basel, Switzerland) (n = 1,065) or placebo (n = 1,062) once yearly. Both groups received a loading dose of vitamin D2 or D3 intramuscularly or orally, followed by 800–1,200 IU vitamin D and 1,000–1,500 mg elemental calcium orally on a daily basis. Concomitant antiresorptive therapy with calcitonin, hormone-replacement therapy, selective estrogen receptor modulators, and tibolone was allowed. All study procedures were approved by the local institutional review board at each participating site.
Outcomes and Follow-Up
Subsequent clinically apparent fractures, including second hip fractures, were the primary end point and were adjudicated by a blinded central committee that reviewed radiographs and radiograph reports.
Potential risk factors for index hip subtrochanteric fracture were determined for each subject. Baseline comorbidities, demographics, and prior osteoporosis therapy were recorded at baseline. Bone mineral density (BMD) was measured at baseline and yearly thereafter. Other measures that were used in the present analysis include EQ-5D total score and each of the separate dimensions, additional fractures after randomization, and survival status. Laboratory values were measured at a central laboratory prior to study drug infusion.
Statistical Analysis
A univariate logistic regression model was used to test if a predictor variable was associated with the index hip subtrochanteric fracture. The model was also used to determine whether place of residence following the index hospitalization was affected by the type of index hip fracture. A Cox regression model stratified by treatment group was used to evaluate whether the incidence rates of subsequent clinical fracture, hip fracture, and death during the study were higher in patients with index hip subtrochanteric fracture compared to other patients. Analysis of covariance models were used to evaluate the between-index hip-fracture type difference in change from baseline EQ-5D visual analogue scale (VAS) and utility scores during the study.
Results
Of the qualifying hip fractures, 5.2% were subtrochanteric, 54.8% femoral neck, 33.0% intertrochanteric, 7.1% other (generally complex fractures of mixed type), and 0.1% missing. Of the 106 subjects in the study who had sustained a baseline qualifying subtrochanteric fracture, 50 were randomly assigned to zoledronic acid 5 mg therapy and 56 to placebo (Table 1).
In examining the baseline characteristics for those with subtrochanteric fractures relative to those who had sustained other types of hip fractures, no significant differences were observed between groups in the majority of factors (Table 2). The percentage of patients with extreme mobility problems (confined to bed) was twofold higher in patients with index subtrochanteric fracture (9.9%) compared with other patients (4.7%). In all, a significantly higher proportion of patients with subtrochanteric fractures at baseline had problems walking (i.e., mobility problems) than did patients with other hip fractures at baseline (82.2 vs. 77.1%, respectively; logistic regression P = 0.05) (Table 2). In total, 4.7% of subjects with subtrochanteric fractures and 5.1% with other hip-fracture types reported taking permitted concomitant osteoporosis treatment at the time of their index fracture (Table 3). However, no patients with index subtrochanteric fractures and only six patients with other types of qualifying hip fractures reported any prior use of bisphosphonates.
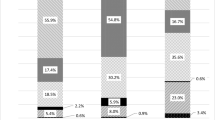
There was no difference in the incidence of death in patients with index subtrochanteric relative to other hip fractures. There was a trend for reduced risk of subsequent fracture over 24 months in those with index subtrochanteric fractures compared with other types of index hip fractures (3.47 vs. 11.61%, hazard ratio [HR] = 0.43, 95% confidence interval [CI] 0.18–1.03; P = 0.05). Patients with index subtrochanteric fractures were also numerically less likely to have a subsequent hip fracture (HR = 0.75, 95% CI 0.18–3.07; P = 0.68) over 24 months than those with other index hip fractures, although the difference was not statistically significant. At the end of the study, the mean increases in EQ-5D VAS scores and utility scores were significantly larger in patients with index subtrochanteric fractures compared to patients with other index hip-fracture types (P = 0.04 and P = 0.001, respectively) (Fig. 1). There was no difference between index hip-fracture types in the proportion of patients with extreme problems in the EQ-5D profile at the end of the study. The only exception was in patients with other types of index hip fracture, who were significantly more likely to have extreme problems with self-care than those with an index subtrochanteric fracture (at baseline, the difference was not statistically significant) (Fig. 2). Patients with index subtrochanteric fractures were numerically less likely than those with other index hip fractures to be discharged to an assisted living facility or nursing home following the index hospitalization (odds ratio 0.90, 95% CI 0.52–1.56), but this difference was not statistically significant (P = 0.70).
Between-hip-fracture type comparison of change from baseline in a EQ-5D Visual Analogue Scale (VAS) and b EQ-5D utility scores by visit (intention-to-treat population). a *P = 0.02, **P = 0.04. b *P = 0.006, **P = 0.025, ***P = 0.001. n number of patients with measurements at both baseline and postbaseline visits, CI confidence interval. Scale 0 corresponds to worst possible health and 100 corresponds to perfect health. Error bars show standard error of least squares mean (LSM). Value at end of study visit is hip-fracture type difference, i.e., the LSM difference of subtrochanteric hip fracture versus other hip fracture on the change from baseline. The P value is obtained from an analysis of covariance model, with baseline score, region, treatment, and hip-fracture type as explanatory variables
In the overall study population, the rate of new hip fractures was 2.0% (23/1,065) in the zoledronic acid group and 3.5% (33/1,062) in the placebo group (P = 0.18). There were two subsequent subtrochanteric fractures reported as adverse events: one in the zoledronic acid group and one in the placebo group. Neither was reported as an atypical fracture with thickened cortices. As a result, no conclusions could be drawn about subtrochanteric fractures in the 24 months post-zoledronic acid therapy in this group of patients with a hip fracture at baseline. Further, in the safety analysis of the overall study population, no adverse effects on the healing of fractures were noted. Specifically, blinded adjudication by a panel of external experts found that there were no differences between treatment groups in delayed fracture healing or nonunion, regardless of fracture type or time of study drug infusion.
Discussion
The long-term safety of bisphosphonates for the treatment of osteoporosis has been questioned. Several case series have suggested a link between prolonged bisphosphonate therapy and atypical fractures [1–5]. In an early report, three patients sustained low-energy nonvertebral fractures of the femoral shaft while receiving long-term alendronate therapy, with bone biopsies showing evidence of severely suppressed bone turnover and fracture healing that was either delayed or absent [1]. Low-energy subtrochanteric fractures were associated with prodromal pain in the affected hip in the months preceding the fall, with a stress reaction demonstrated in the cortex in the contralateral femur [4] and a simple, transverse fracture with a unicortical beak in an area of cortical hypertrophy often seen associated with alendronate use [2]. A unique radiographic pattern, defined as a simple transverse or oblique (≤30°) fracture with beaking of the cortex and diffuse cortical thickening of the proximal femoral shaft, has been reported with cortical thickening present in the contralateral femur in all patients with this pattern [3].
In a more recent study examining atypical fractures of the subtrochanteric or diaphyseal femur, it was reported that these fractures were rare and that in this (albeit underpowered) study, no significant increase in risk was seen in association with bisphosphonate use for as long as 10 years [6]. In addition, the results from a register-based national cohort study and cross-sectional analysis conducted in Denmark did not support previous suggestions of increased risk of atypical subtrochanteric fractures as a consequence of long-term bisphosphonate use [9].
Little is known about the epidemiology of typical, osteoporosis-related subtrochanteric fractures in older adults. A better understanding of the risk factors, incidence, and outcomes of these fractures is useful to frame the debate about atypical, bisphosphonate-related subtrochanteric fractures. Prior studies have suggested that subtrochanteric fractures make up approximately 5–10% of hip fractures in older adults [10]. The incidence appears to be higher in Asians than in other populations, and this has been hypothesized to relate to higher prevalence of osteomalacia [11]. It is not clear whether risk factors or outcomes of these fractures differ from other types of hip fracture.
The HORIZON-RFT, as a large-scale, randomized, controlled trial in a high-risk population who had already sustained a hip fracture and including a subset of patients with an index subtrochanteric fracture, provided a unique framework for meaningful data on bisphosphonate use and osteoporosis-related subtrochanteric fracture risk. Data were systematically collected at the time of study entry, and it was observed that 5.2% of patients had sustained an index subtrochanteric fracture; all were bisphosphonate-naive. Unfortunately, we were not able to determine whether these patients exhibited the thickened cortices associated with atypical subtrochanteric fractures. Subjects with an index subtrochanteric fracture were significantly more likely to report baseline mobility problems than those with other types of index hip fractures and were likely to have significantly greater increases in EQ-5D VAS and utility scores (i.e., greater declines in condition). These findings suggest that subtrochanteric fractures might be associated with greater postoperative mobility limitations and poorer health outcomes overall than other hip-fracture types. Although the results suggest that mobility problems might be a risk factor for subtrochanteric fractures, some inconsistencies in the data, for example, that patients with other types of hip fracture were significantly more likely to have extreme problems with self-care at the end of the study, suggest that this could be a chance finding, particularly since only univariate analysis was performed.
Over the course of the study, only two subtrochanteric fractures occurred; and once again, we could not determine whether these were atypical subtrochanteric fractures with thickened cortices. However, our study adds to the understanding of subtrochanteric fractures as a type of osteoporotic hip fracture with similar risk factors and outcomes to intertrochanteric and femoral neck fractures. Additional studies are needed to accurately distinguish “atypical” fractures possibly associated with bisphosphonate use from the more common subtrochanteric fracture related to osteoporosis.
Our study is not without limitations. Our greatest limitation was that the subtrochanteric fractures were “reported,” without knowledge of time of occurrence, and not reviewed by a radiologist to confirm that they were indeed subtrochanteric fractures, a similar limitation identified in the post-hoc analysis recently published by Black and colleagues using data from the HORIZON-RFT, FIT and FLEX trials [6]. Unfortunately, both the baseline and follow-up X-rays are not readily available for review. As a result, the fractures could not be fully characterized. In addition, there was no central reading of the index hip fractures. However, despite some limitations, this analysis provides important additional information on the incidence and risk of subtrochanteric fractures.
Conclusions
Our post-hoc analysis demonstrated that osteoporosis-related subtrochanteric fractures are not uncommon and do occur in bisphosphonate-naive patients. In patients with recent hip fracture, extreme problems with mobility may have been a predisposing risk factor to the development of incident subtrochanteric fractures. Finally, the incidence of subtrochanteric fractures post-zoledronic acid therapy was rare and too small to draw any meaningful conclusions about the occurrence of new subtrochanteric fractures with short-term zoledronic acid therapy.
References
Odvina CV, Zerwekh JE, Rao DS, Maalouf N, Gottschalk FA, Pak CY (2005) Severely suppressed bone turnover: a potential complication of alendronate therapy. J Clin Endocrinol Metab 90:1294–1301
Neviaser AS, Lane JM, Lenart BA, Edobor-Osula F, Lorich DG (2008) Low-energy femoral shaft fractures associated with alendronate use. J Orthop Trauma 22:346–350
Lenart BA, Lorich DG, Lane JM (2008) Atypical fractures of the femoral diaphysis in postmenopausal women taking alendronate. N Engl J Med 358:1304–1306
Goh SK, Yang KY, Koh JS, Wong MK, Chua SY, Chua DT, Howe TS (2007) Subtrochanteric insufficiency fractures in patients on alendronate therapy: a caution. J Bone Joint Surg Br 89:349–353
Lyles KW, Colón-Emeric CS, Magaziner JS, Adachi JD, Pieper CF, Mautalen C, Hyldstrup L, Recknor C, Nordsletten L, Moore KA, Lavecchia C, Zhang J, Mesenbrink P, Hodgson PK, Abrams K, Orloff JJ, Horowitz Z, Eriksen EF, Boonen S (2007) Zoledronic acid and clinical fractures and mortality after hip fracture. N Engl J Med 357:1799–1809
Black DM, Kelly MP, Genant HK, Palermo L, Eastell R, Bucci-Rechtweg C, Cauley J, Leung PC, Boonen S, Santora A, de Papp A, Bauer DC (2010) Bisphosphonates and fractures of the subtrochanteric or diaphyseal femur. N Engl J Med 362:1761–1771
Colón-Emeric C, Kuchibhatla M, Pieper C, Hawkes W, Fredman L, Magaziner J, Zimmerman S, Lyles KW (2003) The contribution of hip fracture to risk of subsequent fractures: data from two longitudinal studies. Osteoporos Int 14:879–883
Klotzbuecher CM, Ross PD, Landsman PB, Abbott TA III, Berger M (2000) Patients with prior fractures have an increased risk of future fractures: a summary of the literature and statistical synthesis. J Bone Miner Res 15:721–739
Abrahamsen B, Eiken P, Eastell R (2009) Subtrochanteric and diaphyseal femur fractures in patients treated with alendronate: a register-based national cohort study. J Bone Miner Res 24:1095–1102
Trikha V, Rastogi S (2010) Epidemiology and rehabilitation of hip fractures in the geriatric population. IJPMR 16:16–19
Calder SJ, Anderson GH, Harper WM, Gregg PJ (1994) Ethnic variation in epidemiology and rehabilitation of hip fracture. BMJ 309:1124–1125
Acknowledgements
The authors wish to thank the patients who took part in the HORIZON-RFT [5], and the investigators and staff at participating clinical centers. The HORIZON-RFT was supported by Novartis Pharma AG; the paper was developed independently by the authors. Editorial assistance was provided by Holly Gilbert-Jones of BioScience Communications.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Additional information
This study is conducted on behalf of the HORIZON-Recurrent Fracture Trial.
Adachi has received research funding and consulting fees from Amgen, Bristol-Myers Squibb, Eli Lilly, GSK, Merck, Novartis, Nycomed, Pfizer, Procter & Gamble, Roche, sanofi-aventis, Servier, Wyeth, and Warner Chilcott. Lyles has received research funding from Amgen, Novartis and Alliance for Better Bone Health, and consulting fees from Amgen, Novartis, Procter & Gamble, Merck, Kirin Pharmaceutical, GTx, Lilly, GSK, Bone Medical Ltd., Wyeth, and Osteologix. He is inventor of US Patent Application: ‘Medication kits and formulations for preventing, treating or reducing secondary fractures after previous fracture’, Number 12532285. He is co-inventor of US Patent Application: ‘Methods for preventing or reducing secondary fractures after hip fracture’, Number 20050272707, and of US Provisional Patent Application: ‘Bisphosphonates and heart disease’. Boonen has received research funding and consulting fees from Novartis. Colón-Emeric has received research funding from Pfizer and Novartis, and consulting fees from Amgen and Novartis. Hyldstrup has received research funding from Eli Lilly, Nycomed, Pfizer and Novo Nordisk and consulting fees from Novartis, Amgen, Eli Lilly, Nycomed, Roche and GSK. Nordsletten has received research funding and consulting fees from Amgen, Novartis, DePuy, Stryker, and Biomet. Pieper has received consulting fees from Novartis. Recknor has received consulting fees from Eli Lilly, Dramatic Health, Novartis, Takeda and Zelos, and lecture fees from Amgen, Novartis and Publicis Meetings. Su is an employee of Novartis. Bucci-Rechtweg is an employee of Novartis and owns stock in the company. Magaziner has received research funding from Eli Lilly, Merck, and Novartis. He has consulted with or served on an advisory board for Amgen, Eli Lilly, GSK, Novartis, and sanofi-aventis.
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Adachi, J.D., Lyles, K., Boonen, S. et al. Subtrochanteric Fractures in Bisphosphonate-Naive Patients: Results from the HORIZON-Recurrent Fracture Trial. Calcif Tissue Int 89, 427–433 (2011). https://doi.org/10.1007/s00223-011-9543-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-011-9543-8