Abstract
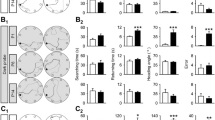
Rodent open field behavior is highly organized and occurs spontaneously in novel environments. This organization is disrupted in mice with vestibular pathology, suggesting vestibular signals provide important contributions to this behavior. A caveat to this interpretation is that previous studies have investigated open field behavior in adult mice with congenital vestibular dysfunction, and the observed deficits may have resulted from developmental changes instead of the lack of vestibular signals. To determine which aspects of open field behavior depend specifically on vestibular signals, mouse movement organization was examined under dark and light conditions at two time points, 1 and 2 months, after bilateral chemical labyrinthectomy. Our results show that acquired vestibular damage selectively disrupted the organization of open field behavior. Access to visual environmental cues attenuated, but did not eliminate, these significant group differences. Improvement in movement organization from the first to the second testing session was limited to progression path circuity. These observations provide evidence for the role of the vestibular system in maintaining spatial orientation and establishes a foundation to investigate neuroplasticity in brain systems that process self-movement information.






Similar content being viewed by others
References
Antoine MW, Vijayakumar S, McKeehan N, Jones SM, Hébert JM (2017) The severity of vestibular dysfunction in deafness as a determinant of comorbid hyperactivity or anxiety. J Neurosci 37(20):5144–5154
Avni R, Zadicario P, Eilam D (2006) Exploration in a dark open field: a shift from directional to positional progression and a proposed model of acquiring spatial information. Behav Brain Res 171(2):313–323
Avni R, Elkan T, Dror AA, Shefer S, Eilam D, Avraham KB, Mintz M (2009) Mice with vestibular deficiency display hyperactivity, disorientation, and signs of anxiety. Behav Brain Res 202(2):210–217
Batschelet E (1981) "Circular statistics in biology." Academic Press, 111 Fifth ave., New York, 10003, 388
Bland BH, Colom LV (1993) Extrinsic and intrinsic properties underlying oscillation and synchrony in limbic cortex. Prog Neurobiol 41(2):157–208
Blankenship PA, Cherep LA, Donaldson TN, Brockman SN, Trainer AD, Yoder RM, Wallace DG (2017) Otolith dysfunction alters exploratory movement in mice. Behav Brain Res 325:1–11
Brandt T, Schautzer F, Hamilton DA, Brüning R, Markowitsch HJ, Kalla R, Darlington C, Smith P, Strupp M (2005) Vestibular loss causes hippocampal atrophy and impaired spatial memory in humans. Brain 128(11):2732–2741
Chen Y-C, Pellis SM, Sirkin DW, Potegal M, Teitelbaum P (1986) Bandage backfall: labyrinthine and non-labyrinthine components. Physiol Behav 37(5):805–814
Clark BJ, Hamilton DA, Whishaw IQ (2006) Motor activity (exploration) and formation of home bases in mice (C57BL/6) influenced by visual and tactile cues: modification of movement distribution, distance, location, and speed. Physiol Behav 87(4):805–816
Coelho CM, Balaban CD (2015) Visuo-vestibular contributions to anxiety and fear. Neurosci Biobehav Rev 48:148–159
Donaldson T, Jennings KT, Cherep LA, McNeela AM, Depreux FF, Jodelka FM, Hastings ML, Wallace DG (2018) Antisense oligonucleotide therapy rescues disruptions in organization of exploratory movements associated with Usher syndrome type 1C in mice. Behav Brain Res 338:76–87
Donaldson T, Jennings K, Cherep L, Blankenship P, Blackwell A, Yoder R, Wallace D (2019) Progression and stop organization reveals conservation of movement organization during dark exploration across rats and mice. Behav Proc 162:29–38
Drai D, Golani I (2001) SEE: a tool for the visualization and analysis of rodent exploratory behavior. Neurosci Biobehav Rev 25(5):409–426
Drai D, Benjamini Y, Golani I (2000) Statistical discrimination of natural modes of motion in rat exploratory behavior. J Neurosci Methods 96(2):119–131
Eilam D, Golani I (1989) Home base behavior of rats (Rattus norvegicus) exploring a novel environment. Behav Brain Res 34(3):199–211
Furman JM, Jacob RG (2001) A clinical taxonomy of dizziness and anxiety in the otoneurological setting. J Anxiety Disord 15(1–2):9–26
Golani I, Benjamini Y, Eilam D (1993) Stopping behavior: constraints on exploration in rats (Rattus norvegicus). Behav Brain Res 53(1–2):21–33
Goodridge JP, Dudchenko PA, Worboys KA, Golob EJ, Taube JS (1998) Cue control and head direction cells. Behav Neurosci 112(4):749
Harvey RE, Rutan SA, Willey GR, Siegel JJ, Clark BJ, Yoder RM (2018) Linear self-motion cues support the spatial distribution and stability of hippocampal place cells. Curr Biol 28(11):1803–1810
Hines DJ, Whishaw IQ (2005) Home bases formed to visual cues but not to self-movement (dead reckoning) cues in exploring hippocampectomized rats. Eur J Neurosci 22(9):2363–2375
Kalueff AV, Ishikawa K, Griffith AJ (2008) Anxiety and otovestibular disorders: linking behavioral phenotypes in men and mice. Behav Brain Res 186(1):1–11
Maaswinkel H, Whishaw IQ (1999) Homing with locale, taxon, and dead reckoning strategies by foraging rats: sensory hierarchy in spatial navigation. Behav Brain Res 99(2):143–152
Maaswinkel H, Jarrard LE, Whishaw IQ (1999) Hippocampectomized rats are impaired in homing by path integration. Hippocampus 9(5):553–561
Muir GM, Brown JE, Carey JP, Hirvonen TP, Della Santina CC, Minor LB, Taube JS (2009) Disruption of the head direction cell signal after occlusion of the semicircular canals in the freely moving chinchilla. J Neurosci 29(46):14521–14533
Muller RU, Kubie JL (1987) The effects of changes in the environment on the spatial firing of hippocampal complex-spike cells. J Neurosci 7(7):1951–1968
Nemati F, Whishaw IQ (2007) The point of entry contributes to the organization of exploratory behavior of rats on an open field: an example of spontaneous episodic memory. Behav Brain Res 182(1):119–128
Oddie SD, Bland BH (1998) Hippocampal formation theta activity and movement selection. Neurosci Biobehav Rev 22(2):221–231
O’Keefe J, Burgess N (1996) Geometric determinants of the place fields of hippocampal neurons. Nature 381(6581):425–428
O'Keefe J, Nadel L (1978) The hippocampus as a cognitive map. Clarendon Press, Oxford
Ossenkopp K-P, Hargreaves EL (1993) Spatial learning in an enclosed eight-arm radial maze in rats with sodium arsanilate-induced labyrinthectomies. Behav Neural Biol 59(3):253–257
Perna G, Dario A, Caldirola D, Stefania B, Cesarani A, Bellodi L (2001) Panic disorder: the role of the balance system. J Psychiatr Res 35(5):279–286
Quirk GJ, Muller RU, Kubie JL (1990) The firing of hippocampal place cells in the dark depends on the rat’s recent experience. J Neurosci 10(6):2008–2017
Rossier J, Haeberli C, Schenk F (2000) Auditory cues support place navigation in rats when associated with a visual cue. Behav Brain Res 117(1–2):209–214
Russell NA, Horii A, Smith PF, Darlington CL, Bilkey DK (2006) Lesions of the vestibular system disrupt hippocampal theta rhythm in the rat. J Neurophysiol 96(1):4–14
Schaeffer A (1928) Spiral movement in man. J Morphol 45(1):293–398
Souman JL, Frissen I, Sreenivasa MN, Ernst MO (2009) Walking straight into circles. Curr Biol 19(18):1538–1542
Stackman RW, Taube JS (1997) Firing properties of head direction cells in the rat anterior thalamic nucleus: dependence on vestibular input. J Neurosci 17(11):4349–4358
Stackman RW, Clark AS, Taube JS (2002) Hippocampal spatial representations require vestibular input. Hippocampus 12(3):291–303
Stackman RW, Golob EJ, Bassett JP, Taube JS (2003) Passive transport disrupts directional path integration by rat head direction cells. J Neurophysiol 90(5):2862–2874
Taube JS, Muller RU, Ranck JB (1990) Head-direction cells recorded from the postsubiculum in freely moving rats. I. Description and quantitative analysis. J Neurosci 10(2):420–435
Tchernichovski O, Golani I (1995) A phase plane representation of rat exploratory behavior. J Neurosci Methods 62(1–2):21–27
Thompson SM, Berkowitz LE, Clark BJ (2018) Behavioral and neural subsystems of rodent exploration. Learn Motiv 61:3–15
Valerio S, Taube JS (2016) Head direction cell activity is absent in mice without the horizontal semicircular canals. J Neurosci 36(3):741–754
Wallace DG, Whishaw IQ (2003) NMDA lesions of Ammon’s horn and the dentate gyrus disrupt the direct and temporally paced homing displayed by rats exploring a novel environment: evidence for a role of the hippocampus in dead reckoning. Eur J Neurosci 18(3):513–523
Wallace DG, Hines DJ, Pellis SM, Whishaw IQ (2002) Vestibular information is required for dead reckoning in the rat. J Neurosci 22(22):10009–10017
Wallace DG, Choudhry S, Martin MM (2006) Comparative analysis of movement characteristics during dead-reckoning-based navigation in humans and rats. J Comp Psychol 120(4):331
Watanabe S, Yoshida M (2007) Auditory cued spatial learning in mice. Physiol Behav 92(5):906–910
Whishaw I, Vanderwolf CH (1973) Hippocampal EEG and behavior: change in amplitude and frequency of RSA (theta rhythm) associated with spontaneous and learned movement patterns in rats and cats. Behav Biol 8(4):461–484
Whishaw IQ, Cassel JC, Majchrzak M, Cassel S, Will B (1994) “Short-stops” in rats with fimbria-fornix lesions: Evidence for change in the mobility gradient. Hippocampus 4(5):577–582
Wiener SI, Taube JS (2005) Head direction cells and the neural mechanisms of spatial orientation, MIT Press
Winter SS, Köppen JR, Ebert TB, Wallace DG (2013) Limbic system structures differentially contribute to exploratory trip organization of the rat. Hippocampus 23(2):139–152
Xie Y, Bigelow RT, Frankenthaler SF, Studenski SA, Moffat SD, Agrawal Y (2017) Vestibular loss in older adults is associated with impaired spatial navigation: data from the triangle completion task. Front Neurol 8:173
Yoder RM, Kirby SL (2014) Otoconia-deficient mice show selective spatial deficits. Hippocampus 24(10):1169–1177
Yoder RM, Taube JS (2009) Head direction cell activity in mice: robust directional signal depends on intact otolith organs. J Neurosci 29(4):1061–1076
Yoder RM, Goebel EA, Köppen JR, Blankenship PA, Blackwell AA, Wallace DG (2015) Otolithic information is required for homing in the mouse. Hippocampus 25(8):890–899
Zheng Y, Darlington CL, Smith PF (2006) Impairment and recovery on a food foraging task following unilateral vestibular deafferentation in rats. Hippocampus 16(4):368–378
Zheng Y, Goddard M, Darlington CL, Smith PF (2009) Long-term deficits on a foraging task after bilateral vestibular deafferentation in rats. Hippocampus 19(5):480–486
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest statement
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Data availability statement
Data will be made available on reasonable request.
Additional information
Communicated by Bill J Yates.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Banovetz, M.T., I Lake, R., Blackwell, A.A. et al. Effects of acquired vestibular pathology on the organization of mouse exploratory behavior. Exp Brain Res 239, 1125–1139 (2021). https://doi.org/10.1007/s00221-020-06032-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-020-06032-1