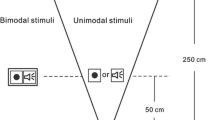
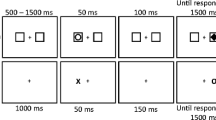
Abstract
Many researchers have taken the Colavita effect to represent a paradigm case of visual dominance. Broadly defined, the effect occurs when people fail to respond to an auditory target if they also have to respond to a visual target presented at the same time. Previous studies have revealed the remarkable resilience of this effect to various manipulations. In fact, a reversal of the Colavita visual dominance effect (i.e., auditory dominance) has never been reported. Here, we present a series of experiments designed to investigate whether it is possible to reverse the Colavita effect when the target stimuli consist of repetitions embedded in simultaneously presented auditory and visual streams of stimuli. In line with previous findings, the Colavita effect was still observed for an immediate repetition task, but when an n-1 repetition detection task was used, a reversal of visual dominance was demonstrated. These results suggest that masking from intervening stimuli between n-1 repetition targets was responsible for the elimination and reversal of the Colavita visual dominance effect. They further suggest that varying the presence of a mask (pattern, conceptual, or absent) in the repetition detection task gives rise to different patterns of sensory dominance (i.e., visual dominance, an elimination of the Colavita effect, or even auditory dominance).


Similar content being viewed by others
Notes
It is important to note that the task used to study the Colavita visual dominance effect has evolved from Colavita’s (1974) simple detection paradigm and expanded to include the recognition of predefined pictures and sounds (see, for example, Ngo et al. 2010; Sinnett et al. 2007). Furthermore, while we believe it appropriate to call visual dominance observed in such tasks the Colavita effect, others may not. For the purposes of the current investigation, we consider the paradigm and stimuli used here to be an expansion of the Colavita visual dominance effect as done so by previous authors (see Spence et al. 2011, for a review).
It should be noted that a reversal of the Colavita visual dominance effect has never been observed in any variant of Colavita’s original visual dominance task when measuring unimodal response error rates to bimodal target stimuli. However, a recent demonstration of multisensory competition using a passive oddball event-related potential (ERP) design suggests that auditory dominance might be evident when measuring the P300 latency. Specifically, Robinson et al. (2010) measured visual and auditory oddball detection in a passive (i.e., no response) task in which the participants were presented with frequent (i.e., standards) and infrequent stimuli (i.e., oddballs) embedded in a stream of auditory and visual events. The main comparison involved the potential modulation of unimodal baseline ERPs under conditions of multisensory presentation, as indicated by an increased or decreased latency of the P300 component. Interestingly, compared to their respective unimodal baseline ERPs, bimodal presentations either slowed down or sped-up the P300. Specifically, the visual P300 was delayed when paired with sounds, whereas the auditory P300 occurred somewhat earlier when paired with pictures. Despite these results suggesting a reversal in visual dominance in favor of auditory dominance, it should be noted that the task utilized by Robinson et al. was markedly different from the traditional Colavita visual dominance task. Additionally, there was no measure of accuracy on bimodal trials. Therefore, a direct comparison of the results of these studies is somewhat problematic (see also Lewkowicz 1988a, b; Robinson and Sloutsky 2004, 2010; Sloutsky and Robinson 2008, for similar results with infant studies).
Note, though, that while many of the Colavita studies still involve simple detection, the complexity of the targets used in these studies have evolved from the simple detection of beeps and lights to the recognition of predefined pictures and sounds (see, for example, Koppen et al. 2008; Ngo et al. 2010; Sinnett et al. 2007).
In Colavita’s (1974) original study, he addressed the question of whether increasing the intensity and saliency of the auditory relative to the visual stimuli would eliminate or reverse the visual dominance effect. Colavita asked participants to subjectively increase the intensity of a tone until it was twice as intense as a light, yet this stimulus modulation had no effect on the magnitude of the Colavita effect.
Between-experiments comparisons (independent-samples t tests) were conducted on the percentages of correct responses and missed targets data from Experiments 1 and 2. These comparisons revealed that participants responded significantly more accurately to visual targets (mean difference = 11.2%, P = .01) and auditory targets (mean difference = 11.6%, P = .05) in Experiment 2 as compared to Experiment 1. There was no difference in the percentage of correct responses for bimodal targets (mean difference = 0.4%) between Experiments 1 and 2, P = .95. The participants also missed significantly fewer visual targets (mean difference = 13.0%; P = .006), auditory targets (mean difference = 12.2%, P = .03), and bimodal targets (mean difference = 12.7, P = .03) in Experiment 2 as compared to Experiment 1.
References
Baddeley AD (1992) Working memory. Philos Trans R Soc B 302:311–324
Baddeley A (2000) The episodic buffer: a new component of working memory? Trends Cogn Sci 4:417–423
Colavita FB (1974) Human sensory dominance. Percept Psychophys 16:409–412
Colavita FB, Weisberg D (1979) A further investigation of visual dominance. Percept Psychophys 25:345–347
Cowan N (1984) On short and long auditory stores. Psychol Bull 96:341–370
Di Lollo V (1977) Temporal characteristics of iconic memory. Nature 267:241–243
Elliot LL (1971) Backward and forward masking. Audiology 10:65–76
Enns JT, Di Lollo V (2000) What’s new in visual masking? Trends Cogn Sci 4:345–352
Intraub H (1984) Conceptual masking: the effects of subsequent visual events on memory for pictures. J Exp Psychol Learn 10:115–125
Koppen C, Spence C (2007a) Assessing the role of stimulus probability on the Colavita visual dominance effect. Neurosci Lett 418:266–271
Koppen C, Spence C (2007b) Audiovisual asynchrony modulates the Colavita visual dominance effect. Brain Res 1186:224–232
Koppen C, Spence C (2007c) Seeing the light: exploring the Colavita visual dominance effect. Exp Brain Res 180:737–754
Koppen C, Spence C (2007d) Spatial coincidence modulates the Colavita visual dominance effect. Neurosci Lett 417:107–111
Koppen C, Alsius A, Spence C (2008) Semantic congruency and the Colavita visual dominance effect. Exp Brain Res 184:533–546
Lessard N, Pare M, Lepore F, Lassonde M (1998) Early-blind human subjects localize sound sources better than sighted subjects. Nature 395:278–280
Lewkowicz DJ (1988a) Sensory dominance in infants: 1. Six-month-old infants’ response to auditory-visual compounds. Dev Psychol 24:155–171
Lewkowicz DJ (1988b) Sensory dominance in infants: 2. Ten-month-old infants’ response to auditory-visual compounds. Dev Psychol 24:172–182
Loftus GR, Ginn M (1984) Perceptual and conceptual masking of pictures. J Exp Psychol Learn 10:435–441
Long GM (1980) Iconic memory: a review and critique of the study of short-term visual storage. Psychol Bull 88:785–820
Massaro DW, Burke D (1991) Perceptual development and auditory backward recognition masking. Dev Psychol 27:85–96
Massaro DW, Cohen MM, Idson WL (1976) Recognition masking of auditory lateralization and pitch judgments. J Acoust Soc Am 59:434–441
Moro SS, Steeves JKE (2010) An absence of the Colavita effect: enhanced multisensory processing in monocular blindness. In: Poster presented at the 11th international multisensory research forum, 16–19th June. Liverpool, UK
Ngo MK, Sinnett S, Soto-Faraco S, Spence C (2010) Repetition blindness and the Colavita effect. Neurosci Lett 480:186–190
Oram MW, Xiao D, Dritschel B, Payne KR (2002) The temporal resolution of neural codes: Does response latency have a unique role? Philos Trans R Soc B 357:987–1001
Oxenham AJ, Moore BCJ (1994) Modeling the additivity of nonsimultaneous masking. J Acoust Soc Am 98:1921–1934
Oxenham AJ, Wojtczak M (2010) Frequency selectivity and masking. In: Plack CJ (ed) The Oxford handbook of auditory science. Oxford University Press, Oxford, pp 5–44
Potter MC (1976) Short-term conceptual memory for pictures. J Exp Psychol Learn 2:509–522
Potter MC (1999) Understanding sentences and scenes: the role of conceptual short-term memory. In: Coltheart V (ed) Fleeting memories: cognition of brief visual stimuli. MIT Press, Cambridge, pp 13–46
Robinson CW, Sloutsky VM (2004) Auditory dominance and its change in the course of development. Child Dev 75:1397–1401
Robinson CW, Ahmar N, Sloutsky VM (2010) Evidence for auditory dominance in a passive oddball task. In: Ohlsson S, Catrambone R (eds) Proceedings of the 32nd annual conference of the cognitive science society. Cognitive Science Society, Austin, pp 2644–2649
Sinnett S, Spence C, Soto-Faraco S (2007) Visual dominance and attention: the Colavita effect revisited. Percept Psychophys 69:673–686
Sloutsky VM, Robinson CW (2008) The role of words and sounds in infants’ visual processing: from overshadowing to attentional tuning. Cogn Sci 32:354–377
Snodgrass JG, Vanderwart M (1980) A standardized set of 260 pictures: norms for name agreement, image agreement, familiarity, and visual complexity. J Exp Psychol Learn 6:174–215
Soto-Faraco S, Spence C (2002) Modality-specific auditory and visual temporal processing deficits. Q J Exp Psychol 55A:23–40
Spence C (2009) Explaining the Colavita visual dominance effect. Prog Brain Res 176:245–258
Sperling G (1960) The information available in brief visual presentations. Psychol Monogr 74:1–29
Thorpe S, Fize D, Marlot C (1996) Speed of processing in the human visual system. Nature 381:520–522
Welch RB, Warren DH (1980) Immediate perceptual response to intersensory discrepancy. Psychol Bull 88:638–667
Welch RB, Warren DH (1986) Intersensory interactions. In: Boff KR, Kaufman L, Thomas JP (eds) Handbook of perception and human performance, vol I. Sensory processes in perception. Wiley, New York, pp 1–36
Acknowledgments
MKN was supported by the Clarendon Fund Scholarship from Oxford University. SS-F was supported by grants SEJ 2007-64103/PSIC and CDS00012 from the Spanish Ministerio de Ciencia e Innovación, ERC StG 263145, and grant 2009SGR-292 from DURSI.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
The percentages of correct responses for all three target types (visual, auditory, and bimodal) were rather low as compared to those values observed in previous studies of the Colavita visual dominance effect (e.g., see Ngo et al. 2010). We suspected that this may have been due to the difficulty that participants experienced in trying to simultaneously divide their attention between the visual and auditory modalities in the present study. In order to assess this possibility, we therefore conducted an additional control experiment in which the participants (N = 10) were instructed to detect only unimodal visual or auditory n-1 repetition targets in separate blocks of experimental trials. The procedure was identical to that used in the main experiment reported in the text, except for the fact that the participants completed five blocks of trials detecting each target modality. The order of presentation of each target modality was counterbalanced across participants. At the start of each block of trials, the participants were instructed to respond to n-1 repetition targets in either the auditory or visual modality by pressing a key on the keyboard.
The percentage of correct responses and the RTs from those trials in which the participants responded correctly for each participant and condition were calculated. Separate paired-samples t tests revealed that participants responded significantly more accurately to auditory targets (M = 79.6%, SE = 4.6%) than to visual targets (M = 67.6%, SE = 4.5%), t(9) = 2.71, P = .02. What is more, the participants detected the visual targets (M = 591 ms, SE = 29 ms) significantly faster than the auditory n-1 targets (M = 608 ms, SE = 34 ms), t(9) = 2.40, P = .04. This may have been due to the fact that the auditory stimuli were time-evolving, whereas the visual stimuli were immediately perceptible. That is, the information needed to identify a naturalistic sound such as a cat’s “meow” may not arrive until relatively late in the presentation of the sound. All of the information contained within a picture, on the other hand, is immediately available as soon as it is presented (and is likely processed after approximately 150 ms of stimulus onset; Oram et al. 2002; Thorpe et al. 1996). It is also possible that there were some speed-accuracy trade-offs that might have been present in the data (see Edwards 1965) given that participants were faster but less accurate at detecting visual, as compared to auditory, targets.
The results of this control experiment therefore confirm that participants found the task much easier when they need only attend to one sensory modality (either vision or audition). This was evidenced by the substantially higher percentages of correct responses (68 and 80% for visual and auditory, respectively) as compared to those observed in Experiment 1 (35 and 56% for visual and auditory targets, respectively), where participants had to divide their attention between the visual and auditory modalities in order to detect either type of target n-1 repetitions in the experiments reported in the main text.
Rights and permissions
About this article
Cite this article
Ngo, M.K., Cadieux, M.L., Sinnett, S. et al. Reversing the Colavita visual dominance effect. Exp Brain Res 214, 607–618 (2011). https://doi.org/10.1007/s00221-011-2859-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-011-2859-9