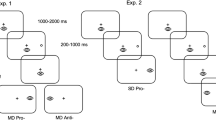
Abstract
Antisaccades require the suppression of a stimulus-driven response (i.e., response suppression) and the computation of a movement plan mirror-symmetrical to the location of a target (i.e., vector inversion). The goal of the present study was to determine whether response suppression, vector inversion or both contribute to previously reported differences in the online control of pro- and antisaccades (Heath in Exp Brain Res 203:743–752, 2010a). Pro- and antisaccades were completed in separate blocks (i.e., blocked schedule) and a block wherein the spatial relation between stimulus and response was provided at response cuing (i.e., random schedule). Notably, the random schedule provides a relative means for equating response suppression across pro- and antisaccades. To examine online trajectory amendments, we computed the proportion of variance (R 2 values) explained by the spatial location of the eye at early, middle and late stages of saccade trajectories relative to the saccade’s ultimate endpoint. The basis for this analysis is that between-task differences in R 2 magnitudes reflect differences in the use of feedback for online trajectory amendments: small R 2 values represent a trajectory supported via online control whereas larger R 2 values reflect a reduction in online control. Results show that antisaccades yielded larger R 2 values than prosaccades from early to late stages of saccade trajectories, and this finding was observed regardless of whether or not tasks were equated for response suppression. Thus, we propose that the intentional nature of vector inversion disrupts the normally online control of saccades and renders a mode of control that is not optimized to support error-reducing trajectory amendments.





Similar content being viewed by others
Notes
As indicated in the “Methods”, trials involving a direction error were placed back in the random trial matrix and rerun. Thus, the number of direction errors reported here represents the average number of error trials (per participant) in each of the blocked and random schedules.
MT data are reported in order to show that differences in endpoint accuracy are unrelated to a speed/accuracy trade-off and to demonstrate that between-task and between-schedule differences in R 2 values are unrelated to movement time differences.
In a previous study, we demonstrated that saccades directed left or right of a central fixation did not influence the spatiotemporal parameters of pro- and antisaccade trajectories (Heath et al. 2010a; see also West et al. 2009). For that reason, space (i.e., targets in left vs. right space) was not included in our ANOVA model.
As a matter of course, we do not compute multi-directional post hoc contrasts. That said, we thought it important to demonstrate that blocked schedule prosaccades elicited faster RTs than any of the other experimental conditions. Thus, comparison of prosaccades across feedback schedules revealed that blocked schedule ones were reliably faster than their random schedule counterparts (t(11) = 6.84, P < 0.001).
Examination of the ANOVA summary for R 2 values revealed F-ratios less than one for schedule, schedule by task, and time by schedule by task. The null effect of blocked and random schedules on saccade trajectories is therefore not tied to an inadequate replication sample size.
References
Becker W, Fuchs AF (1969) Further properties of the human saccadic system: eye movements and correction saccades with and without visual fixation points. Vision Res 9:1247–1258
Binsted G, Heath M (2004) Can the motor system utilize a stored representation to control movement? Behav Brain Sci 27:25–27
Binsted G, Brownell K, Vorontsova Z, Heath M, Saucier D (2007) Visuomotor system uses target features unavailable to conscious awareness. Proc Natl Acad Sci USA 104:12669–12672
Brainard DH (1997) The psychophysics toolbox. Spat Vis 10:433–436
Bridgeman B, Lewis S, Heit G, Nagle M (1979) Relation between cognitive and motor-oriented systems of visual position perception. J Exp Psychol Hum Percept Perform 5:692–700
Brown MR, DeSouza JF, Goltz HC, Ford K, Menon RS, Goodale MA, Everling S (2004) Comparison of memory- and visually guided saccades using event- related fMRI. J Neurophysiol 91:873–889
Brown MR, Goltz HC, Vilis T, Ford KA, Everling S (2006) Inhibition and generation of saccades: rapid event-related fMRI of prosaccades, antisaccades, and nogo trials. Neuroimage 33:644–659
Connolly JD, Goodale MA, Desouza JF, Menon RS, Vilis T (2000) A comparison of frontoparietal fMRI activation during anti-saccades and anti-pointing. J Neurophysiol 84:1645–1655
Dafoe JM, Armstrong IT, Munoz DP (2007) The influence of stimulus direction and eccentricity on pro- and anti-saccades in humans. Exp Brain Res 179:563–570
Deubel H (1995) Separate adaptive mechanisms for the control of reactive and volitional saccadic eye movements. Vision Res 35:3529–3540
Doma H, Hallett PE (1988) Dependence of saccadic eye-movements on stimulus luminance, and an effect of task. Vision Res 28:915–924
Elliott D, Binsted G, Heath M (1999) The control of goal-directed limb movements: correcting errors in the trajectory. Hum Mov Sci 18:121–136
Evdokimidis I, Tsekou H, Smyrnis N (2006) The mirror antisaccade task: direction- amplitude interaction and spatial accuracy characteristics. Exp Brain Res 174:304–311
Everling S, Spantekow A, Krappmann P, Flohr H (1998) Event-related potentials associated with correct and incorrect responses in a cued antisaccade task. Exp Brain Res 118:27–34
Fischer B, Weber H (1992) Characteristics of “anti” saccades in man. Exp Brain Res 89:415–424
Fitts PM (1954) The information capacity of the human motor system in controlling the amplitude of movement. J Exp Psychol 47:381–391
Ford KA, Goltz HC, Brown MR, Everling S (2005) Neural processes associated with antisaccade task performance investigated with event-related FMRI. J Neurophysiol 94:429–440
Gaveau V, Martin O, Prablanc C, Pélisson D, Urquizar C, Desmurget M (2003) Online modification of saccadic eye movements by retinal signals. Neuroreport 14:875–878
Gaveau V, Pélisson D, Blangero A, Urquizar C, Prablanc C, Vighetto A, Pisella L (2008) Saccade control and eye-hand coordination in optic ataxia. Neuropsychologia 46:475–486
Glover S, Dixon P (2001) Motor adaptation to an optical illusion. Exp Brain Res 137:254–258
Goodale MA (2010) Transforming vision into action. Vision Res (in press)
Goodale MA, Pelisson D, Prablanc C (1986) Large adjustments in visually guided reaching do not depend on vision of the hand or perception of target displacement. Nature 320:748–750
Hallett PE (1978) Primary and secondary saccades to goals defined by instructions. Vision Res 18:1279–1296
Heath M (2005) Role of limb and target vision in the online control of memory-guided reaches. Mot Control 9:281–311
Heath M, Rival C, Binsted G (2004a) Can the motor system resolve a premovement bias in grip aperture? Online analysis of grasping the Müller-Lyer illusion. Exp Brain Res 158:378–384
Heath M, Westwood DA, Binsted G (2004b) The control of memory-guided reaching movements in peripersonal space. Mot Control 8:76–106
Heath M, Maraj A, Gradkowski A, Binsted G (2009a) Anti-pointing is mediated by a perceptual bias of target location in left and right visual space. Exp Brain Res 192:275–286
Heath M, Maraj A, Maddigan M, Binsted G (2009b) The antipointing task: vector inversion is supported by a perceptual estimate of visual space. J Mot Behav 41:383–392
Heath M, Dunham K, Binsted G, Godbolt B (2010a) Antisaccades exhibit diminished online control relative to prosaccades. Exp Brain Res 203:743–752
Heath M, Neely KA, Krigolson O, Binsted G (2010b) Memory-guided reaching: what the visuomotor system knows and how long it knows it. In: Elliott D, Khan M (eds) Vision and goal-directed movement: neurobahevioral perspectives. Human Kinetics, Champaign, pp 79–96
Krappmann P, Everling S, Flohr H (1998) Accuracy of visually and memory-guided antisaccades in man. Vision Res 38:2979–2985
Krigolson O, Clark N, Heath M, Binsted G (2007) The proximity of visual landmarks impacts reaching performance. Spat Vis 20:317–336
Maraj A, Heath M (2010) Antipointing: perception-based visual information renders an offline mode of control. Exp Brain Res 202:55–64
McDowell JE, Dyckman KA, Austin BP, Clementz BA (2008) Neurophysiology and neuroanatomy of reflexive and volitional saccades: evidence from studies of humans. Brain Cogn 68:255–270
Messier J, Kalaska JF (1999) Comparison of variability of initial kinematics and endpoints of reaching movements. Exp Brain Res 125:139–152
Moon SY, Barton JJ, Mikulski S, Polli FE, Cain MS, Vangel M, Hämäläinen MS, Manoach DS (2007) Where left becomes right: a magnetoencephalographic study of sensorimotor transformation for antisaccades. Neuroimage 36:1313–1323
Munoz DP, Everling S (2004) Look away: the anti-saccade task and the voluntary control of eye movement. Nat Rev Neurosci 5:218–228
Olk B, Kingstone A (2003) Why are antisaccades slower than prosaccades? A novel finding using a new paradigm. Neuroreport 14:151–155
Pedhazur EJ (1997) Multiple regression in behavioral research: explanation and prediction. Harcourt Brace Publishers, Orlando, FL
Pisella L, Gréa H, Tilikete C, Vighetto A, Desmurget M, Rode G, Boisson D, Rossetti Y (2000) An ‘automatic pilot’ for the hand in human posterior parietal cortex: toward reinterpreting optic ataxia. Nat Neurosci 3:729–736
Prablanc C, Martin O (1992) Automatic control during hand reaching at undetected two-dimensional target displacements. J Neurophysiol 67:455–469
Robinson DA (1964) The mechanics of human saccadic eye movement. J Physiol 174:245–264
Rossetti Y, Revol P, McIntosh R, Pisella L, Rode G, Danckert J, Tilikete C, Dijkerman HC, Boisson D, Vighetto A, Michel F, Milner AD (2005) Visually guided reaching: bilateral posterior parietal lesions cause a switch from fast visuomotor to slow cognitive control. Neuropsychologia 43:162–177
Sparks DL, Holland R, Guthrie BL (1976) Size and distribution of movement fields in the monkey superior colliculus. Brain Res 113:21–34
West GL, Welsh TN, Pratt J (2009) Saccadic trajectories receive online correction: evidence for a feedback-based system of oculomotor control. J Mot Behav 41:117–127
Wurtz RH (2008) Neuronal mechanisms of visual stability. Vision Res 48:2070–2089
Wurtz RH, Goldberg ME (1972) Activity of superior colliculus in behaving monkey. IV. Effects of lesions on eye movements. J Neurophysiol 35:587–596
Zhang M, Barash S (2000) Neuronal switching of sensorimotor transformations for antisaccades. Nature 408:971–975
Acknowledgments
This work was supported by a grant from the Natural Sciences and Engineering Research Council of Canada and a Major Academic Development Fund from the University of Western Ontario.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Heath, M., Weiler, J., Marriott, K. et al. Vector inversion diminishes the online control of antisaccades. Exp Brain Res 209, 117–127 (2011). https://doi.org/10.1007/s00221-010-2525-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-010-2525-7