Abstract
Fiber rich by-products derived from primary agri-food production such as carrot pomace and potato pulp are available in large quantities, but their functional properties do not necessarily meet the requirements for use in specific food applications. Thermomechanical treatment (extrusion) of carrot pomace and potato pulp changes both dietary fiber polysaccharide structures and technofunctionality of the materials. Solubility of dietary fiber constituents changes, resulting in higher levels of water- and ethanol-soluble poly-/oligosaccharides. On a structural level, particularly arabinans and galactans as neutral side chains of type I rhamnogalacturonan were degraded under thermomechanical stress. Galacturonic acid portions (preferably from homogalacturonan or rhamnogalacturonan I) and their degree of methylation were also negatively affected. On a functional level, water absorption of potato pulp increased up to three times following extrusion, whereas water absorption of carrot pomace decreased with extrusion processing. The observed, enhanced swelling behavior for extruded carrot pomace was accompanied by higher complex viscosity of the dispersions. Swelling of potato pulp particles increased largely (up to 25 times) following extrusion, resulting in highly viscous pastes. Phytochemicals were retained up to 50%, heat-induced contaminants were formed only to a small extent (up to 8.1 mg 5-hydroxymethylfurfural·kg− 1 dry matter for carrot pomace; up to 71 µg acrylamide·kg− 1 dry matter for potato pulp).
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dietary fiber, which is mostly composed of non-digestible polysaccharides and oligosaccharides, is known to be beneficial in human nutrition [1]. Diseases such as diabetes mellitus type II are associated with a low-fiber diet [2]. Contrarily, specific dietary fiber constituents can contribute to a positive regulation of blood glucose and cholesterol levels [3, 4]. In addition, dietary fiber intake is closely related to gut health, among other, by positively affecting gut microbiota composition [5, 6]. Therefore, the European Food Safety Authority (EFSA) generally recommends the daily consumption of 25 g of dietary fiber [7], with other recommendations being even higher (IoM [8], up to 38 g for men under age 50). Consequently, there is a trend to enrich various foods with dietary fiber without compromising food quality. Besides their nutritional properties, dietary fiber constituents may offer functional properties such as water binding or gelation [9,10,11]. However, both nutritional and technological functions strongly differ, depending on the composition and structural details of the fiber constituents.
Incorporation of food by-products derived from primary agri-food production, which are rich in dietary fiber (mostly plant cell wall polysaccharides, but also associated compounds such as lignin and proanthocyanidins), in products such as bread, pasta, and cookies has been widely studied in the last decades [11]. Most often, the by-products were incorporated without further processing. However, several previous studies have shown that thermal and/or mechanical processing modifies the functional properties of by-products [10]. For example, extrusion was shown to be a suitable process to increase the water solubility of food by-products and increase the water holding capacity or viscosity. As a result, syneresis in food products such as deserts and sausages can be decreased or the texture of bread be improved [10]. We recently demonstrated that the functional properties of, for example, apple and chokeberry pomace are strongly affected by extrusion [10, 12,13,14]. On a polysaccharide structural level, extrusion of these food by-products mainly degraded arabinans as neutral side chains of rhamnogalacturonan I [12, 13].
Whether or not these findings can easily be transferred to other raw materials remains to be answered. Carrot pomace and potato pulp dietary fiber differs from apple pomace fiber mainly in the composition of pectic polysaccharides. Neutral rhamnogalacturonan I side chains of carrot pomace contain arabinans and galactans in approximately equal proportions [15], whereas pectic side chains of potato pulp are rich in galactans [16]. Additionally, potato pulp often contains large portions of residual starch [16, 17], which may affect functional properties, too. Moreover, these raw materials are characterized by their contents of low-molecular weight phytochemicals, with carotenoids being most important for carrot pomace, and chlorogenic acids for potato pulp [1, 9]. Due to material temperatures > 100 ℃ during extrusion, the retention of these nutritionally valuable, but heat-labile substances may be a concern [18, 19]. In addition to a potential degradation of beneficial compounds, toxic phytochemicals such as glycoalkaloids (in potato pomace) and heat-induced contaminants of toxicological concern, such as 5-hydroxymethylfurfural (HMF) and acrylamide, can be present or formed. Acrylamide formation may be especially relevant for potato pulp due to its high levels of asparagine.
Therefore, the aim of this study was to characterize compositional and structural changes of carrot pomace (from juice production) and potato pulp (from starch production) with emphasis on (dietary fiber) polysaccharides, phytochemicals, and heat-induced contaminants caused by extrusion. Additionally, changes of functional properties of the extruded samples were monitored.
Materials and methods
Material
Potato pulp was obtained from Emsland-Stärke GmbH (Emlichheim, Germany). Carrot pomace was kindly provided by GNT Europe GmbH (Aachen, Germany). The residual moisture contents were 10.3 ± 0.2% and 5.7 ± 0.1% (g·100 g−1), respectively (determined by Karl-Fischer titration; Titroline alfa, Schott Instruments GmbH, Germany). Commercially available carrot chips were purchased online. Untreated and extruded materials were milled for 30 s (coffee mill M55, Petra Electric, Jettingen-Scheppach, Germany) and sieved to a particle size of 0.14 < x < 0.28 mm. The obtained powder was dried at 25 ℃ and 8 mbar in a vacuum dryer (VO101, Memmert GmbH + Co. KG, Schwabach, Germany) to constant mass.
Chemicals
Thermostable α-amylase Termamyl 120 L (EC 3.2.1.1, from Bacillus licheniformis, ≥500 U·mg-1 protein (biuret)), amyloglucosidase (EC 3.2.1.3, from Aspergillus niger, 260 U·mL−1), and protease Alcalase 2.4 L (EC 3.4.21.62, from Bacillus licheniformis, 2.4 AU·g−1; all from Sigma Aldrich, St. Louis, Missouri) were used for preparative dietary fiber isolation. A thermostable α-amylase (EC 3.2.11, from Bacillus licheniformis, 3000 U·mL−1) and an amyloglucosidase (EC 3.2.1.3, from Aspergillus niger, 36000 U·g-1; both from Megazyme, Bray, Ireland) were used in the approaches to analyze dietary fiber analysis and starch. In addition, a protease (EC 3.4.21.14, from Bacillus licheniformis, 9 U·mg-1; Megazyme, Bray, Ireland) was used in the analytical fiber approach. Driselase (from Basidiomycetes) was from Sigma-Aldrich (St. Louis, Missouri). All chemicals and reagents were of analytical grade and purchased from Carl Roth (Karlsruhe, Germany), Merck (Darmstadt, Germany), Sigma-Aldrich (St. Louis, Missouri), or VWR (Radnor, Pennsylvania).
Extrusions processing
Extrusion trials were carried out on a laboratory-scale co-rotating twin-screw extruder (Process 11 Hygenic Twin-Screw Extruder, Thermo Fisher Scientific, Karlsruhe, Germany). The extruder had an L·D−1 ratio of 40 and a die of 2 mm (diameter). The screw configuration was a combination of a kneading zone (3 L·D−1) followed by two reverse elements (for detailed schematic illustration see [20], screw configuration 4). The barrel zone consists of eight sections, which can be heated and cooled separately. The barrel temperatures were adjusted to Tbarrel1 = 40 ℃, Tbarrel2 = 60 ℃, Tbarrel3 = 80 ℃, and Tbarrel4-8 = 100 ℃. Throughout the experiment, the material (0.6 kg∙h− 1, 0.8 kg∙h− 1, 0.9 kg∙h− 1) was fed in the first section, whereas water was added at the second section by a piston-membrane pump (0.4 kg∙h−1, 0.2 kg∙h− 1). Screw speeds of 200 min− 1, 600 min− 1, and 1000 min− 1 were applied.
The material temperature TM of melt was measured in the die adapter. Specific mechanical energy (SME) was calculated as:
where n and nmax are the actual and maximum screw speed (1000 min−1), Md and Md,unload are the actual and idle torque (%), \({\dot{m}}\) represents the total mass flow (kg·h−1), and Pmax the maximum engine power (1.5 kW).
Samples were collected when the conditions were at steady state. For storage, samples were dried for 30 min at 40 ℃.
Basic compositional analysis
Protein contents were analyzed using the Kjeldahl approach with ammonia being detected by an ammonia sensitive electrode (nitrogen conversion factor 6.25) [21]. Ash contents were determined after incineration for 4 h at 500 ℃. Starch was analyzed quantitatively following degradation into its glucose monomer units [12]. In brief, after enzymatic hydrolysis using a thermostable α-amylase and amyloglucosidase, liberated glucose was determined by high-performance anion exchange chromatography with pulsed amperometric detection (HPAEC-PAD; Thermo Fisher Scientific, Waltham, Massachusetts). Starch was calculated as the sum of anhydroglucose. Mono- and disaccharides were determined after aqueous extraction with ultrasonic treatment (four times) using HPAEC-PAD (Thermo Fisher Scientific, Waltham, Massachusetts) as described in detail in [12]. pH was measured with a pH electrode (500 mg of sample in 5 mL of water). To compare data, the pH of recently analyzed apple pomace and its extrudates [12, 13] were also recorded (Table S2).
Dietary fiber analysis
To determine contents of insoluble dietary fiber (IDF), soluble dietary fiber (SDF), and low-molecular weight soluble dietary fiber (LMW-SDF), a combination of the methods AOAC 985.29 [22] and AOAC 2009.01 [23] was applied. The principle of the method is based on enzymatic digestion of starch and proteins by applying a sequence of thermostable α-amylase, protease, and amyloglucosidase. SDF and LMW-SDF were differentiated according to their (in)solubility in 80% ethanol. Ethanol-soluble LMW-SDF was obtained from the filtrate after precipitation of SDF and analyzed by size-exclusion chromatography (Hitachi, Merck, Darmstadt, Germany) with refractive index (RI) detection (Knauer, Berlin, Germany) using a TSKgel PWxl guard column (40 mm × 6.0 mm, particle size 12 µm, Tosoh, Tokyo, Japan) and two size-exclusion columns in a row (TSKgel G2500PWxl, 300 mm × 7.8 mm, particle size 13 µm, Tosoh, Tokyo, Japan) [12]. IDF and SDF contents were corrected for residual protein [21] and ash contents.
Preparative dietary fiber isolation
Preparative isolation of IDF and SDF was carried out following the principles of AOAC method 985.29 using an enzymatic treatment (thermostable α-amylase, protease, and amyloglucosidase) as described by Bunzel et al. [24].
Molecular weight distribution
SDF was dissolved in 50 mM sodium nitrate (2 g·L−1) and treated for 24 h at 40 °C. The supernatant was used for the separation of oligo-/polysaccharides based on their hydrodynamic volumes using HPLC-RI (Hitachi, Merck, Darmstadt, Germany) equipped with a guard column (TosohTSK-gel PWxl 40 mm × 6.0 mm, particle size 12 μm, Tosoh, Tokyo, Japan) and two size-exclusion columns in series (TosohTSK-gel G6000PWxl 300 mm × 7.8 mm, particle size 13 μm; TSKgel G4000PWxl 300 mm × 7.8 mm, particle size 10 μm, Tosoh, Tokyo, Japan). Sodium nitrate was used as eluent at a flow rate of 0.5 mL·min−1 at 50 ℃. Dextrans were used for calibration [13].
Polysaccharide analysis
Monomer composition
The monomer composition of dietary fiber polysaccharides was determined by HPAEC-PAD (Thermo Fisher Scientific, Waltham, Massachusetts) after sulfuric acid hydrolysis (IDF) [25] or methanolysis followed by trifluoroacetic acid (TFA) hydrolysis (SDF) [26]. To determine the monomer composition of the oligosaccharides of LMW-SDF, they were semi-preparatively separated from mono- and disaccharides by HPLC-RI, followed by TFA based hydrolysis of the glyosidic linkages [12].
Methylation analysis
Methylation analysis of IDF, SDF and LMW-SDF poly-/oligosaccharides was performed as previously published [27]. In brief, methylation was performed twice with methyl iodide in DMSO/NaOH. Extraction was done using dichloromethane. Methylated poly-/oligosaccharides were hydrolyzed using TFA, reduced with sodium borodeuteride, and acetylated with acetic anhydride using 1-methylimidazole as a catalyst. Identification of the partially methylated alditol acetates (PMAAs) was performed by gas chromatography (GC) and mass spectrometric (MS) detection. PMAAs were semi-quantitatively calculated using GC coupled to a flame ionization detector applying molar response factors [28].
Arabinan and galactan profiling
Detailed characterization of neutral rhamnogalacturonan I side chains of SDF and IDF polysaccharides was carried out following partial hydrolysis using endo-arabinanase and endo-galactanase as described previously [29]. Liberated oligosaccharides were determined semi-quantitatively by HPAEC-PAD (Thermo Fisher Scientific, Waltham, Massachusetts).
Degree of (pectin) esterification
Polymer-bound galacturonic acid contents were analyzed spectrophotometrically using the Blumenkrantz procedure [30]. To calculate the degree of esterification of IDF and SDF pectins, quantitative 1H-NMR spectroscopy was performed [31]. A suspension of 20 mg sample in 1.5 mL of 2 M NaOH (in D2O) was ultrasonicated for 2 h. Trimethylsilylpropanoic acid-d4 (0.5 mg·mL−1) was added as internal standard. A standard 1H-NMR pulse program (zg30) was used on a Bruker Avance 500 MHz instrument (Ettlingen, Germany), 32 scans were acquired with 65,536 data points and an acquisition time of 3.28 s, and a relaxation delay of 35 s were applied. The degree of methylation and acetylation was calculated related to uronic acid contents.
Analysis of potential heat-induced contaminants
HMF was determined as previously published [32, 33], applying minor modifications. Sample material (50 mg) was shaken in 900 µL of water for 30 min at 800 rpm. Carrez I (150 g·L−1 K4[Fe(CN)6]∙3H2O) and Carrez II (300 g·L−1 ZnSO4∙7H2O) solutions (50 µL each) were successively added, and samples were vortexed and centrifuged. Supernatants were passed through a syringe filter (regenerated cellulose, 0.45 µm) and analyzed by HPLC with diode-array detection (DAD; Shimadzu, Kyõto, Japan). A Luna C18(2) (250 × 4.6 mm, particle size 5 µm; Phenomenex, Torrance, California) column was used, and water (A) and acetonitrile were applied as eluents in linear gradients (0 min 95% A, 35 min 75% A, 40 min 0% A, 45 min 0% A, 46 min 95% A, 50 min 95% A). The flow rate was 1 mL·min−1. Calibration was performed externally in the range of 5–200 µM.
Acrylamide contents were analyzed externally by LC–MS/MS according to an accredited method (DIN EN 16618:2015-06).
Analysis of low-molecular weight phytochemicals
Carotenoids
Carotenoid contents were analyzed according to Abdel-Aal and coworkers (1996) [34]. Sample material (1 g) was weighed into a brown tube, mixed with 1 mL of Driselase suspension (10 mg·mL−1) and 1 mL of water, and allowed to stand overnight under nitrogen. Ethyl acetate (7 mL) was added, and the mixture was left in the dark for 1 h at room temperature after brief vortexing. After vortexing again, the mixture was left in the dark for another 30 min at room temperature. After centrifugation, the supernatant was removed, and extraction was repeated using 3 mL of ethyl acetate and allowing the mixture to stand for 1 h. After centrifugation, the supernatants were combined and made up to 10 mL with ethyl acetate. After filtration (PTFE, 0.45 µm), extracted carotenoids were analyzed by HPLC–DAD (Beckman, Brea, California) on a ProntoSIL C30 column (250 × 4.6 mm, particle size 5 µm; Bischoff, Leonberg, Germany). Methanol/methyl tert-butyl ether/water (A, 81/15/4) and methanol/methyl tert-butyl ether (10/90) each containing 0.1% butylhydroxytoluene were used as eluents. The gradient was as follows: 0 min 100% A, 20 min 40% A, 35 min 40% A, 40 min 100% A, 45 min 100% A. The flow rate was 1.2 mL·min−1, the temperature was hold at 30 ℃, and the eluent was monitored at 450 nm. Calibration was performed using standard compounds (α-carotene, β-carotene) dissolved in ethyl acetate with 0.1% butylhydroxytoluene, and concentrations were determined spectrophotometrically at 446 nm (α-carotene) and 448 nm (β-carotene) after evaporation and dissolving in n-hexane [35, 36].
Glycoalkaloids
Previously published procedures were modified to analyze glycoalkaloids [37, 38]. Potato pulp sample (37 mg) was mixed with 5% acetic acid, vortexed, shaken for 15 min at room temperature, centrifuged, and the supernatant was collected. The extraction procedure was repeated three times. Combined supernatants were filled up to a volume of 5 mL and filtered (PTFE, 0.45 µm). Quantification was performed by using UHPLC (Shimadzu, Kyõto, Japan) electro spray ionization (ESI) MS (LCMS 2020, Shimadzu, Kyõto, Japan). Samples were loaded onto a Luna Omega Polar C18 column (150 × 2.1 mm, particle size 1.6 µm; Phenomenex, Torrance, California) using water (A) and acetonitrile each containing 0.1% formic acid as eluents. The linear gradient was composed as follows: 0.1 min 74% A, 7.0 min 74% A, 8 min 72% A, 9 min 10% A, 12 min 10% A, 13 min 74% A, 15 min 74% A. The flow rate was 0.5 mL·min−1, the temperature was held at 40 °C. ESI–MS detection was performed in positive selected ion monitoring (SIM) mode (4.5 kV) at a nebulization gas flow (nitrogen) of 1.5 L·min−1 and 20 L·min−1 drying gas. The interface temperature was set to 350 °C, the desolvation line temperature was 300 °C, and the heating block was operated at 250 ℃. Glycoalkaloids were detected by monitoring their [M + H]+ ions (m/z 868.4 α-solanine and m/z 852.4 α-chaconine).
Chlorogenic acid
Chlorogenic acid (5-caffeoylquinic acid) contents in the potato samples were determined by UHPLC-DAD (Knauer, Berlin, Germany) according to the methods of Ceymann and coworkers (2011) [39] and Malec and coworkers (2014) [40]. Sample (0.5 g) was weighed into a brown tube, and 3.75 mL of methanol containing 5% formic acid was added. After 15 min of ultrasonic treatment (35 kHz) at 30 ℃, the sample was vortexed and centrifuged. Immediately before measurement, an aliquot of the supernatant (750 µL) was evaporated, and the dried residue was dissolved in 100 µL of water/acetonitrile (97/3) containing 2% formic acid and analyzed on a Kinetex C 18(2) (150 × 4.6 mm, particle size 2.6 µm; Phenomenex, Torrance, California) column at 40 ℃. Eluents to form a linear gradient (flow rate 1 mL·min−1) were water (A) and acetonitrile each with 2% formic acid (0 min 97% A, 5 min 97% A, 25 min 92% A, 45 min 74% A, 52 min 40% A, 54 min 0% A, 59 min 0% A, 61 min 97% A, 68 min 97% A). Detection was carried out at 320 nm.
Water solubility index (WSI) and water absorption index (WAI)
WSI and WAI were determined similar to Anderson et al. [41]. Untreated and extruded materials (0.5 g) were vortexed for 30 s and mixed for 24 h with demineralized water (19.5 g). For WSI determination, tubes were centrifuged (4250×g, 50 min, 25 ℃), and for WAI determination, tubes were not centrifuged but rested 1 h for sedimentation before separation of supernatant and precipitate. Supernatant and precipitate were dried at 80 ℃, normal pressure for 72 h and weighed. WSI and WAI were calculated according to the following equations:
Rheological properties
To prepare dispersions, the ground, sieved, and dried materials (1 g) were mixed with demineralized water (10 g) and stirred on a magnetic mixer (200 min−1) for 10 min, sealed with parafilm and rested for 50 min. Rheological properties were analyzed using an Anton Paar Rheometer MC 301 (Graz, Austria) with a parallel plate geometry (50 mm) and a measurement gap of 1.5 mm. First, samples rested for 90 s in the geometry, afterwards the samples were sheared (oscillatory) at 25 ℃ with an amplitude of 0.1% and a frequency of 1 Hz (within the linear viscoelastic region). Over a time period of 90 s, ten data points were measured and the average was calculated. The shown complex viscosity η* is a mean value of nine measurement points.
Scanning electron microscopy (SEM)
Extruded, milled, sieved and dried samples were fixed with platinum. A LEO 1530 (Carl Zeiss, Oberkochen, Germany) scanning electron microscope at high vacuum was used to observe the particles. All images were taken at an operating voltage of 3 kV.
Microscopic swelling behavior
Image processing using a light microscope Eclipse LV100ND (Nikon, Tokyo, Japan) was performed to observe the swelling of particles (0.14 < x < 0.28 mm) in demineralized water for 90 min with a 20-fold magnification. The particle size was evaluated using ImageJ, whereby the particles were circled by hand.
Statistics
Quantitative data including data from WAI/WSI determinations and rheological measurements are presented as mean ± standard deviation (n = 3). Statistical analyses were performed for quantitative data using OriginPro 2020 9.7.0.188. Differences among samples were tested for statistical significance by using ANOVA, followed by Tukey test (α = 0.05). Semiquantitative structural analyses of isolated IDF, SDF, and LMW-SDF (molecular weight distribution, monosaccharide composition, methylation analysis, arabinan and galactan profiling) were performed in duplicate and are given as mean ± half range (n = 2). Also, experiments to analyze the microscopic swelling behavior were performed in duplicate.
Results and discussion
Compositional analysis of the starting material
Protein, ash, starch, free mono- and disaccharide contents (dry matter basis), and pH of the applied carrot pomace and potato pulp samples are shown in Table 1 and Table S1. The high starch content of potato pulp and the larger contents of mono- and disaccharides in carrot pomace as compared to potato pulp are highlighted with respect to data presented below.
As shown in Fig. 1, the potato pulp dietary fiber fraction is largely dominated by IDF (IDF: 54.2 g·100 g−1 dm; SDF: 9.2 g·100 g−1 dm, LMW-SDF was not detected; reported on a dry matter (dm) basis and to be discussed in more detail later). As we used a thermostable α-amylase to determine dietary fiber contents, the contribution of resistant starch to dietary fiber can be excluded. This approach was chosen because we were interested in the fate of non-starch polysaccharides as dietary fiber contents and less in the fate of starch. This is because the contribution of starch to dietary fiber in the final food products (in which the food by-products are used as ingredients) depends on several parameters, which cannot be mimicked in its entirety here. IDF also dominates in carrot pomace (37.4 g·100 g−1 dm); however, the SDF content (26.7 g·100 g−1 dm) is higher than in potato pulp and even some LMW-SDF was detected in the starting material (1.9 g·100 g−1 dm). Contents of 13–23 g·100 g−1 dm were previously described for SDF of carrot pomace, too [1, 42, 43].
Dietary fiber contents (g·100 g− 1 dry matter (dm), mean ± standard deviation, n = 3) of untreated and extruded carrot pomace and potato pulp samples. The extrusion conditions varied in overall water content (wH2O) and screw speed (n), the barrel temperature was set to 100 ℃ resulting in different specific mechanical energies (SME) and material temperatures (TM). Different letters for each parameter (IDF, SDF, LMW-SDF) and food by-product indicate significant differences (ANOVA, Tukey test, α = 0.05). IDF insoluble dietary fiber, SDF soluble dietary fiber, LMW-SDF low-molecular weight soluble dietary fiber
Finally, it should be noted that the raw materials that were used for the extrusion experiments differed in their water contents (potato pulp: 10.3%; carrot pomace: 5.7%).
Influence of extrusion parameters on the extent of thermomechanical treatment
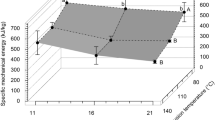
As expected, SME increased with increasing screw speed for both materials and decreased with increasing water contents (Fig. 2). For carrot pomace and a water content of 43%, the increase of screw speed from 200 to 1000 min−1 led to an increase of SME from 73.5 to 253.8 Wh.kg−1. By reducing the water content to 25%, the SME increased by ~ 100 Wh kg−1 at each screw speed. For potato pulp, a significant increase in SME with a reduction in water content from 46 to 28% was observed, too. Material temperatures showed similar tendencies for increasing screw speeds and decreasing water contents. For example, the material temperature of carrot pomace increased at both water contents by 15 ℃ when the screw speed was increased from 200 to 1000 min−1. Generally, under comparable conditions, higher SME values and material temperatures were determined for potato pulp as compared to carrot pomace. Based on the demonstrated variability of SME and material temperatures at different conditions, three samples of each starting material were chosen for a detailed chemical and functional analysis. These samples comprised: carrot pomace: (a) water content (wH2O) 25%, screw speed (n) 200 min− 1; (b) wH2O = 25%, n = 1000 min− 1; (c) wH2O 43%, n = 1000 min− 1; potato pulp: (a) wH2O = 28%, n = 200 min− 1; (b) wH2O = 28%, n = 600 min− 1; (c) wH2O = 46%, n = 600 min−1 (black bordered symbols, Fig. 2).
Dependency of the specific mechanical energy input (SME) (A) and material temperature (TM) (B) on screw speed (n) for potato pulp (triangles) and carrot pomace (quadrangles) for various overall water contents (wH2O) and a barrel temperature (TB) of 100 ℃; total feed rate (\(\dot{m}\)): 1 kg∙h− 1. Extrusion conditions that were chosen to perform an in-depth chemical/functional analysis are marked by black bordered symbols
Influence of extrusion on the morphology of particle surfaces
Scanning electron microscope images showed that particles of the starting material of carrot pomace had a rougher surface as compared to the surface of potato pulp particles (Fig. 3). No apparent alterations of the surface of the carrot pomace particles after extrusion processing were observed. Neither an increase in screw speed nor higher water contents modified the surface of carrot pomace particles. In contrast, the surface of the potato extrudate particles became rougher with increasing thermomechanical treatment, which was observed for apple pomace, too [12, 13].
Compositional data as affected by extrusion
Compositional data (protein, ash, starch, free mono- and disaccharide contents, pH) demonstrated that thermomechanical treatment by extrusion has less of an impact on the parameters analyzed here for both by-products, with the exception of the sugar composition (Table 1; dietary fiber data are discussed in more detail below). The sucrose content of carrot pomace decreased sharply (by 3.3 g·100 g−1 dm) under maximum stress (wH2O = 25%, n = 1000 min− 1). In contrast, contents of glucose and fructose increased suggesting the hydrolytic cleavage of the glycosidic linkage of sucrose during extrusion. Thus, although reaction of the reducing monosaccharides can be expected during extrusion (e.g., in the Maillard reaction) their contents increase with increasing thermomechanical stress.
Contents of dietary fiber and its composition as affected by extrusion
In general, a decrease of IDF contents of carrot pomace and potato pulp was observed due to thermomechanical treatment, whereas contents of SDF increased (Fig. 1). This is in line with previous data obtained from extrusion of apple pomace [12, 13, 44] and for heat-treated carrots, where an increased pectin solubility was shown for blanched carrots [45]. Under maximum thermal and mechanical stress (wH2O = 25%, n = 1000 min− 1), carrot pomace contained even more SDF than IDF. An increase of SDF was also evident as a consequence of the extrusion of potato pulp, with the highest extent at maximum SME (wH2O = 28%, n = 600 min− 1). However, for potato pulp, the content of SDF did not consistently increase with increasing SME but other (unknown) factors appear to be a factor, too. LMW-SDF contents did not change significantly for carrot pomace, whereas in case of potato pulp LMW-SDF contents could be detected following treatment at higher screw speed (n = 600 min−1).
The results clearly indicate solubilization of dietary fiber during the extrusion processing; however, the extent of solubilization appears to be dependent on the individual extrusion parameters. Extrusion-based solubilization can also be suggested from the molecular weight distribution of the water-soluble material, which, for both by-products, became distinctly broader after extrusion (Fig. 4). However, both increase of SDF and broadening of the molecular weight range may also, at least partially, be explained by the formation of Maillard reaction products. The additional peak at approximately 15 mL (> 670 kDa) for carrot pomace (Fig. 4) may indicate the formation of such products by extrusion, which, however, cannot be confirmed for potato pulp, as a distinct peak at 14 mL was already seen in the untreated sample.
Molecular weight distribution of soluble dietary fiber (SDF) polymers of untreated and extruded carrot pomace and potato pulp as determined by high pressure liquid chromatography with refractive index detection, applying dextrans for calibration (gray lines, 5–670 kDa). The extrusion conditions vary in overall water content (wH2O) and screw speed (n) at a barrel temperature of 100 ℃ resulting in different specific mechanical energies (SME) and material temperatures (TM)
Composition of dietary fiber non-starch polysaccharides as affected by extrusion
Insoluble dietary fiber polysaccharides
The main monosaccharide of the non-starch polysaccharides of the untreated food by-products liberated after acid hydrolysis of IDF is glucose (Fig. 5, Table S3).
Monosaccharide composition (mol%) of insoluble dietary fiber (IDF, n = 2), soluble dietary fiber (SDF, n = 2), and low-molecular weight soluble dietary fiber (LMW-SDF, n = 3) of untreated and extruded carrot pomace and potato pulp. The extrusion conditions vary in overall water content (wH2O) and screw speed (n) at a barrel temperature of 100 ℃ resulting in different specific mechanical energies (SME) and material temperatures (TM)
This suggested, in conjunction with data from methylation analysis (Table S4, the presence of cellulose, which is composed of β-1,4-glucopyranose units. The portion of the PMAA of 1,4-glucopyranose was determined to be 56.0 mol% for carrot pomace and 50.2 mol% for potato pulp. Glucose as a monomer can also be released from xyloglucans, which consist of β-1,4-linked glucopyranose units largely substituted with xylose at position O-6 [46]. PMAAs of 1,4,6-glucopyranose and terminal xylose units were detected for carrot pomace and potato pulp, suggesting the presence of xyloglucans. Additional hemicelluloses such as xylans and mannans were also identified in both by-products based on the identification of PMAAs resulting from 1,4-xylopyranose (carrot pomace: 5.4 mol% and potato pulp: 1.8 mol%,) and 1,4-mannopyranose (carrot pomace: 5.7 mol%, and potato pulp: 1.8 mol%) units. Overall, water insoluble hemicelluloses tend to be present as minor components only, whereas pectins appear to be the second dominant insoluble polysaccharides next to cellulose in both by-products. Pectins are dominantly composed of homogalacturonan and rhamnogalacturonan I, with the latter bearing oligo-/polymeric arabinan and (arabino)galactan side chains. High portions of pectic monomers such as arabinose (carrot pomace: 9.7 mol% and potato pulp: 10.3 mol%), galactose (carrot pomace: 14.7 mol% and potato pulp: 35.0 mol%,), and galacturonic acid (carrot pomace: 9.0 mol% and potato pulp: 7.9 mol%) were liberated after acidic hydrolysis. The 1,4-linked galacturonic acid units of homogalacturonan are partially substituted with methyl and/or acetyl groups. For carrot pomace IDF, the portion of methyl esterified galacturonic acid (57%) and the degree of acetylation (78%) was higher as compared to potato pulp (35% degree of methylation, 38% degree of acetylation; Table S7). Different from homogalacturonan, the backbone of rhamnogalacturonan I additionally contains alternating 1,2-linked rhamnopyranose units, which can be substituted at position O-4 with aforementioned arabinans and/or (arabino-)galactans. The ratio of rhamnose to galacturonic acid was 1:4 for both the untreated carrot pomace and the potato pulp, indicating almost similar portions of homogalacturonan and rhamnogalacturonan I. However, IDF pectins of the raw materials differ in their composition of neutral type I rhamnogalacturonan side chains. Potato pulp pectins are rich in galactans as demonstrated by higher galactose (35 mol%) than arabinose (10.3 mol%) contents, being consistent with literature data [16, 17]. Carrot pomace pectins contain more comparable portions of arabinose and galactose and, therefore, less divergent portions of arabinans and galactans as previously reported [15]. Structural details were obtained from methylation analysis. Arabinan side chains consist of 1,5-linked arabinofuranose units that can be substituted at position O-2 and/or position O-3. Almost equal portions of 1,3,5-arabinofuranose (2.4 mol%) and 1,5-arabinofuranose (3.3 mol%) units were determined for carrot pomace IDF (Table S4), indicating highly branched arabinans, with branches being almost exclusively in position O-3. This was confirmed by an arabinan profiling approach [29]. After incubation of carrot pomace IDF with endo-arabinanase, those oligosaccharides that are exclusively linked in position O-3 (total 23.8 mol%) (A-4a, A-6a, A-7b, Fig. S1, Table S5) dominated the liberated, branched oligosaccharides. Only smaller amounts (total 11.1 mol%) of oligosaccharides linked in position O-2 (A-4b, A-7a, Fig. S1, Table S5) were observed. In addition, highly branched oligomers were identified, for example A-7a, which contains neighboring backbone units that are substituted in position O-3 and in both positions O-2 and O-3, respectively. In contrast, arabinans of potato pulp pectins appear to be more linear and less branched in position O-3 (2.9 mol% 1,3,5-arabinofuranose vs. 10.6 mol% 1,5-arabinofuranose units), which was confirmed by our arabinan profiling approach (Table S5). Galactans are composed of 1,4-galactopyranose units that can contain terminal and/or internal 1,4-linked arabinopyranose units [47]. Galactan structures of both raw materials appeared to be quite similar. PMAAs resulting from 1,4-linked galactopyranose units were detected in IDF of carrot pomace and potato pulp; however, in the past, we often observed an underestimation of this PMAA. This was clearly evident from the data of potato pulp IDF. In total, the portion of PMAAs resulting from arabinose units was 18.6 mol% versus 15 mol% for PMAAs resulting from galactose units (Table S4), although galactose largely dominates over arabinose in the monomer composition of potato pulp IDF after sulfuric acid hydrolysis (Fig. 5). Incubation of IDF with endo-galactanase (Table S6, Fig. S1) showed that galactans of both raw materials contain internal 1,4-linked arabinopyranose units, which cannot be differentiated from 1,5-linked arabinofuranose units by methylation analysis.
Soluble dietary fiber polysaccharides
The monomer composition of potato pulp SDF polysaccharides is dominated by galactose, whereas a more balanced ratio between galacturonic acid, arabinose, and galactose, all of which being pectin monomers, was found for carrot pomace (Fig. 5, Table S3). Structures of SDF pectins of both fiber sources appear to be widely comparable to those of IDF, although a difference exist. For example, a higher galactose (28.6 mol%) to arabinose (24.6 mol%) ratio was found for carrot pomace SDF pectins as compared to carrot pomace IDF pectins (Fig. 5, Table S3).
Dietary fiber oligosaccharides
LMW-SDF oligosaccharides from untreated carrot pomace were mainly composed of arabinose, followed by glucose and mannose (Fig. 5, Table S3). Arabinose appears to stem from arabino-oligosaccharides, which were mainly linear with a few branches in position O-3 (Table S4). Surprisingly large portions of mannose (17.8 mol%) and glucose (28.0 mol%) were present in the LMW-SDF. Glucose was potentially released from either malto-oligosaccharides or cello-oligosaccharides as degradation products of starch or cellulose, respectively. Although malto-oligosaccharides may theoretically stem from incomplete starch degradation during the dietary fiber isolation, this does not appear to be the most likely option because comparable oligosaccharides were not identified in potato pulp fiber fractions that contained much larger starch contents than carrot pomace. Analysis by 1H-NMR spectroscopy to investigate the anomeric configuration of the oligomer bound glucose units was not successful because the concentrations were too low for this analytical approach.
Extrusion-based structural modifications of dietary fiber polysaccharides
Structural modifications during extrusion processing are judged by the interpretation of data from the monomer compositional analysis, methylation analysis, and both arabinan and galactan profiling approaches. Thus, it should always be kept in mind that data are normalized to 100 mol%. As a result, the portions of specific monomers or structural elements may be increased, often being misinterpreted as a synthesis of the structural elements or polymers that contain these monomers during extrusion. However, the certainly more likely explanation is that due to degradation of specific polysaccharides or structural elements increased portions of the remaining/unaltered polysaccharides are calculated.
Carrot pomace IDF polysaccharides contained distinctly lower portions of arabinose and galactose (liberated after sulfuric acid hydrolysis) with increasing thermomechanical treatment (Fig. 5). As portions of the individual PMAAs did neither drastically nor consistently change, methylation analysis data suggest that arabinans (see Fig. 6) and galactans were degraded mostly independently of their fine structures as an effect of thermomechanical treatment.
Portions of arabinose-related partially methylated alditol acetates (mol%) resulting from methylation analysis of insoluble dietary fiber (IDF; mean ± half range, n = 2) and soluble dietary fiber (SDF; mean ± half range, n = 2) from untreated and extruded carrot pomace and potato pulp. The extrusion conditions vary in overall water content (wH2O) and screw speed (n) at a barrel temperature of 100 ℃ resulting in different specific mechanical energies (SME) and material temperatures (TM). t terminal, p pyranose, f furanose, Ara arabinose
Results of our arabinan screening (Table S5) basically agree with this; however, portions of highly substituted structures as represented by compound A-7a decreased with increasing thermomechanical treatment and, thus, appear to be more labile. Potato pulp IDF showed an extrusion-based decrease in the portions of galactose, especially at higher screw speed (600 min− 1), indicating a degradation of galactans as neutral pectic polysaccharides. These findings are in accordance with our data on apple pomace pectins that also showed decreasing pectin complexity as a consequence of the extrusion processing [12, 13]. Therefore, extrusion processing appears to alter more extensively the side chains of rhamnogalacturonan I (as compared to other dietary fiber polysaccharides), that is, arabinans and galactans, depending on which neutral side-chain dominates the pectin fraction.
Furthermore, changes in the pectin backbone were indicated by a decrease in the portions of galacturonic acid under maximal thermal and mechanical stress (carrot pomace: wH2O = 25%, n = 1000 min−1; potato pulp: wH2O = 28%, n = 600 min−1). Also related to galacturonic acid, the ratio of rhamnose to galacturonic acid changed in the maximum stressed samples. For carrot pomace, it decreased from 1:4 in the untreated material to 1:6, suggesting higher portions of homogalacturonan than rhamnogalacturonan I in this extrudate. In contrast, for potato pulp, the rhamnose to galacturonic acid ratio increased from 1:4 in the untreated material to 1:2.5, suggesting a more distinct degradation of homogalacturonan as compared to rhamnogalacturonan I. Finally, the degree of pectin esterification decreased from 57 to 29% for carrot pomace (depending on the extrusion conditions) and from 35 to 12% for potato pulp (with increasing thermomechanical energy input; Table S7). The degree of acetylation remained constant due to extrusion for carrot pomace and decreased with increasing speed (n = 600 min−1) from 38% up to 19% (wH2O = 46%) or 12% (wH2O = 28%) depending on the water content for potato pulp (Table S7).
Possible pectin solubilization was not reflected in the SDF monosaccharide composition of the extrudates (Fig. 5, Table S3). However, because solubilized polysaccharides can a) be sufficiently stable or b) further be degraded, either into LMW-SDF or into degradation products that eluded the analyses performed here, the monomer composition of this fraction is a somewhat hard to judge parameter. But, analyses of glycosidic linkages within the polysaccharides suggest that arabinan structures were further depolymerized, at least when treated under highest stress: the portions of arabinose-related PMAAs decreased in carrot pomace (wH2O = 25%, n = 1000 min−1; Fig. 6). Degradation of highly branched arabinan oligomers was also confirmed by the arabinan profiling method (Table S5). In contrast, no hints for extrusion-based arabinan degradation in SDF of potato pulp were detected (Fig. 6). Also, changes in galactan structures were not observed with the methods used here.
Although extrusion processing resulted in an overall increase of LMW-SDF oligosaccharides in the carrot pomace samples, the monomer composition after extrusion was comparable (Fig. 5, Table S3). Increasing arabinose ratios (54.3 mol% up to roughly 67 mol%) are in line with the described solubilization and further degradation of arabinans. Following maximum thermomechanical treatment, small portions of galactose (3.6 mol%) were liberated after acidic hydrolysis of LMW-SDF, indicating further degradation of solubilized galactan structures during extrusion processing at maximum stress.
In contrast, LMW-SDF was only detected after extrusion of potato pulp at a screw speed of 600 min−1, but not at 200 min−1. The oligosaccharides that were generated at wH2O = 46% and n = 600 min−1 contained mainly arabinose, glucose, galactose, and galacturonic acid as monomers (Fig. 5). Methylation analysis suggests the predominant liberation of linear 1,5-linked arabino-oligosaccharides and 1,4-linked galacto-oligosaccharides (Table S4). Again, the origin of 1,4-linked glucose units in this fraction cannot be clarified, although cellulose would be assumed as the most likely source. Increased SME (sample at wH2O = 28% and n = 600 min−1) resulted in the generation of oligosaccharides, in which galacturonic acid was no longer detected. Again, the composition of the liberated LMW-SDF fractions supports our claim that mainly pectins are degraded during extrusion. However, the origin of glucose remains to be clarified in future studies.
Technofunctionality as affected by extrusion
Particle surface modifications (in case of potato pulp) and both partial solubilization and further degradation of solubilized polysaccharides were expected to affect the technofunctionality of the food by-products such as hydration properties (WSI/WAI), swelling behavior, and rheological properties.
Hydration properties
The comparably low WSI of untreated potato pulp (5.3%) was increased by extrusion processing up to 26.2% depending on SME (Fig. 7A, Fig. S2A) and TM (Fig. 7B, Fig. S2B). This may be the result of two factors (a) a high amount of residual starch in the pulp that was modified during extrusion processing (see Table 1) and (b) the solubilization of non-starch polysaccharides as also demonstrated by the increase of SDF and LMW-SDF after extrusion processing (Fig. 1). It has been demonstrated earlier that the WSI of potato starch increases with increasing thermomechanical treatment due to the destruction of starch granules thereby increasing starch solubility [48]. The WSI of untreated carrot pomace was higher (30.6%) as compared to the WSI of untreated potato pulp, which is also reflected by the higher content of SDF and LMW-SDF in the untreated carrot pomace (see Fig. 1). An SME/material temperature dependent increase of WSI was also found for carrot pomace. However, a distinct increase was only seen under the harshest conditions analyzed here. These data reflect well the solubilization of dietary fiber polysaccharides, which is the most important factor for carrot pomace due to negligible starch contents.
The WAI of untreated potato pulp (8.8 g·g−1) was also much lower as compared to untreated carrot pomace (32.4 g·g−1) (Fig. 8, Fig. S3). Extrusion processing of potato pulp increased the WAI up to 3 times. However, both increasing SME and increasing material temperature resulted in decreased WAI values, potentially due to destruction of insoluble particles and starch polymers, although a fragmentation of starch was not apparent from the analyzed starch contents (see Table 1). However, it is possible that partial starch fragmentation resulted in oligosaccharides that were still captured in the analytical determination of starch. Different from potato pulp, the WAI values of carrot pomace generally decreased after extrusion processing. The sample that was extruded at a water content of 43% showed a surprisingly low WAI value, which cannot be explained by the solubility and the composition of the dietary fiber polysaccharides (Fig. 1). As the surface morphology of this sample did not differ from the other samples, and an influence of particle size fraction was excluded by the usage of the particle size fraction of 0.14–0.28 µm, the low WAI value of this sample cannot be explained at this time. Another porosity might be an explanation and will be investigated in the following.
Swelling behavior
The size of untreated carrot pomace particles increased by ~ 11 times of their initial size when particles were allowed to swell for 90 min (Fig. 9A). A drastic decrease of particle growth was observed for carrot samples that were extruded with a water content of 43% at screw speed of 1000 min−1, potentially reflecting the low WAI value of this sample. However, a similar swelling behavior (increase of particle size by ~ 3 time after 90 min) was observed for carrot samples that were extruded with 25% water content at 1000 min−1 and that had a much higher WAI value than the sample extruded at a water content of 43%. After extrusion processing with 25% water content at screw speed of 200 min−1 a particle growth of ~ 8 times was observed after 90 min. Thus, although varying in its magnitude, extrusion processing resulted in reduced swelling of the particles, which cannot simply be explained by a reduced water absorption of the samples. Again, extrusion processing had an opposite effect on the properties of potato pulp. Whereas the particle size of untreated potato pulp changed only slightly (increase of particle size by ~ 2 times after 90 min) during the swelling experiment, a significant increase in size was observed for extruded potato pulp samples (Fig. 9B). The particle size of samples extruded with 46% water content at screw speed of 600 min−1 increased by 27.6 compared to particle size of untreated material, tantamount to the maximum swelling of potato pulp particles achieved here. An increase in thermomechanical treatment, or brief in the SME, resulted in reduced swelling capacity, which, however, was still larger as compared to the untreated material. Different from carrot pomace, these findings are in accordance with the results for WAI. The higher the water absorption, the more distinctive the particle growth. The decrease in swelling might be explained by the increase in thermomechanical treatment, which might destruct the insoluble particles as well as starch, and thus a less porous matrix remains and in consequence less water can be absorbed [49].
Rheological properties
Data for the complex viscosity of aqueous dispersions of carrot pomace are shown in Fig. 10. The complex viscosity of untreated carrot pomace was higher (~ 606 Pas) as compared to extruded samples, for which a decrease of the complex viscosity was observed depending on conditions applied. Just as seen for WAI and swelling the sample that was extruded at a water content of 43% showed the lowest complex viscosity, too.
Rheological measurements of potato pulp were not feasible due to water release while adjusting the rheometer gap. To compare the rheological behavior, pictures of the water pulp dispersions were taken (Fig. 11). Untreated material formed a liquid-like dispersion showing that it was not able to absorb all added water. In contrast, the sample that was extruded at a water content of 46% formed a very firm, solid-like dispersion (no free water was observed), as well as the sample that was extruded at a comparable SME (wH2O = 28%, n = 200 min−1). However, increasing SME (by increasing the screw speed to 600 min−1 at a water content of 28%) resulted in products that produced noticeable softer dispersions. Thus, the sample with the lowest WAI and the lowest tendency to swell also showed the lowest complex viscosity.
Profiles of selected phytochemicals as affected by extrusion processing
As demonstrated so far, both the profile of dietary fiber and the technofunctional properties of food by-products can be altered using extrusion. However, the food by-products may contain low-molecular weight phytochemicals that were not exhaustively extracted during the first use of the potatoes and carrots, respectively. From a nutritional point of view these phytochemicals can be either beneficial, for example, carotenoids or chlorogenic acid, or detrimental, for example, glycoalkaloids. Thus, knowledge about their fate during extrusion processing is of great interest. Data on phytochemicals of both untreated carrot pomace and potato pulp are presented in Table 2.
Contents of α- and β-carotenes were reduced by up to roughly 50% during extrusion processing, which is in line with data on β-carotene contents as affected by extrusion published earlier [18, 19]. The SME largely affected the contents of intact carotenoids after extrusion processing. Similarly, contents of chlorogenic acid in in potato pulp decreased by almost up to 50% as a consequence of extrusion processing (Table 2). Just as demonstrated for the carotenoids, increasing SME reduces the amount of intact chlorogenic acids in the potato pulp. (Partial) instability of (poly)phenolic compounds during extrusion has also been detected following thermomechanical treatment of chokeberry pomace [50] and buckwheat [51], respectively.
As expected contents of glycoalkaloids in potato samples were not reduced during extrusion processing (Table 2). Glycoalkaloids are commonly considered quite stable under the influence of heat [52, 53]. In general, glycoalkaloid levels vary depending on factors such as potato variety and storage conditions [37, 54, 55]. Both α-solanine and α-chaconine were determined in all samples analyzed here, with α-chaconine being dominant. This can be explained by the fact that potato pulp contains higher portions of peel components than mesocarp, and that the peel contains higher levels of α-chaconine [37, 54, 55]. Total contents of glycoalkaloids increased with increasing thermomechanical treatment. Because formation of glycoalkaloids during extrusion can be excluded, it is rather suggested that glycoalkaloids become more accessible for analytical extraction through extrusion processing.
The German Federal Institute for Risk Assessment (BfR) recommended that total glycoalkaloid content in fresh potatoes should not exceed 100 mg·kg−1 fresh weight, thus avoiding to surpass the no observed adverse effect level of 0.5 mg glycoalkaloids·kg−1 body weight per day. This value is based on an average consumption of 350 g of potatoes, potato products and dishes per day [56]. However, as extruded potato pulp would be used in lower amounts as a food ingredient and because the values determined here are based on a dry weight basis, a direct comparison of these recommendations with the levels determined here is difficult.
Formation of food-borne contaminants during extrusion processing
Although extrusion processing clearly enhances the options to use carrot pomace and potato pulp as ingredients (depending on the extrusion parameters as demonstrated above), excessive formation of food-borne contaminants during extrusion might limit their applicability. HMF, a heat-induced contaminant, which is prone to be formed in samples that are rich in mono- and disaccharides, was already found in untreated carrot pomace (4.1 mg·kg−1 dm), potentially as a result of the drying process of this by-product. With increasing thermomechanical treatment, HMF levels initially decreased, but increased to 8.1 mg·kg−1 dm at maximum stress (Table 3).
To the best of our knowledge, there were no studies on HMF in carrot pomace so far. We analyzed commercially available, thermally treated carrot chips to compare with carrot pomace. The analyzed carrot chips contained 6.6 ± 1.1 mg HMF·kg− 1 dm, which is comparable to HMF contents analyzed for carrot pomace samples. Therefore, the potential formation of HMF during extrusion conditions applied in this study does not appear to limit the use of extruded carrot pomace in food products. HMF was neither detected in the untreated potato pulp sample nor in the extrudates, which can be well explained by the low content of free mono- and disaccharides in the pulp.
In contrast to HMF formation, acrylamide formation did not occur in significant amounts during the extrusion of carrot pomace because the analyzed levels were below the limit of quantitation in all samples analyzed in this study (Table 3). However, untreated potato pulp already contained 41 µg·kg−1 dm acrylamide. The acrylamide level decreased slightly to 35 µg·kg− 1 dm when a water content of 46% was used during extrusion processing. Increasing thermomechanical stress resulted in an increase up to 71 µg·kg−1 dm. Thus, although quantifiable, the acrylamide formation appears to be comparably low, resulting in acrylamide contents below the benchmark levels in foodstuffs (for example, breakfast cereals: 300 µg·kg− 1) according to Article 1 in conjunction with annex IV of the Commission regulation (EU) 2017/2158 [57]. Also, it has to be considered that the extrudates are not consumed as a product on their own, but as food ingredients.
In conclusion, extrusion is a promising method to modify the functional properties of carrot and potato based by-products. Technofunctional changes are accompanied by structural changes of dietary fiber polysaccharides. Larger contents of soluble dietary fiber polysaccharides were detected, with pectic polysaccharides being the most susceptible target for structural, extrusion-based modifications. Here, the neutral side chains of rhamnogalacturonan I appear to be mostly affected. Retention of potentially beneficial phytochemicals can be partially achieved; process induced contaminants were formed, but the levels do not to be of major concern. However, extrusion-based functionalization can only be generalized to a limited extent, since it strongly depends on the overall composition of the starting material, with, for example, remaining starch being an important factor. Also, controlling the pH during extrusion may be a useful tool to tune the extrusion-based modifications in a targeted manner; however, details on the role of pH need to be analyzed in future studies.
Abbreviations
- BfR:
-
German Federal Institute for Risk Assessment
- DAD:
-
Diode-array detector
- Dm:
-
Dry matter
- EFSA:
-
European Food Safety Authority
- ESI:
-
Electro-spray ionization
- GC:
-
Gas chromatography
- HMF:
-
5-Hydroxymethylfurfural
- HPAEC:
-
High-performance anion exchange chromatography with pulsed amperometric detection
- HPLC:
-
High-performance liquid chromatography
- IDF:
-
Insoluble dietary fiber
- LMW-SDF:
-
Low-molecular weight soluble dietary fiber
- LOD:
-
Limit of detection
- LOQ:
-
Limit of quantification
- MS:
-
Mass spectrometry
- PMAA:
-
Partially methylated alditol acetate
- RI:
-
Refractive index
- SDF:
-
Soluble dietary fiber
- SIM:
-
Selected ion monitoring
- SME:
-
Specific mechanical energy
- TFA:
-
Trifluoroacetic acid
- WAI:
-
Water absorption index
- WSI:
-
Water solubility index
References
O’Shea N, Arendt EK, Gallagher E (2012) Dietary fibre and phytochemical characteristics of fruit and vegetable by-products and their recent applications as novel ingredients in food products. Innov Food Sci Emerg Technol 16:1–10. https://doi.org/10.1016/j.ifset.2012.06.002
Schweizer TF, Würsch P (1991) The physiological and nutritional importance of dietary fibre. Experientia 47:181–186. https://doi.org/10.1007/BF01945423
deVries JW, Camire ME, Cho S et al (2001) The definition of dietary fiber. Cereal Food World 46:112–126
Slavin J (2013) Fiber and prebiotics: mechanisms and health benefits. Nutrients 5:1417–1435. https://doi.org/10.3390/nu5041417
Holscher HD (2017) Dietary fiber and prebiotics and the gastrointestinal microbiota. Gut Microbes 8:172–184. https://doi.org/10.1080/19490976.2017.1290756
Larsen N, Bussolo de Souza C, Krych L et al (2019) Potential of pectins to beneficially modulate the gut microbiota depends on their structural properties. Front Microbiol 10:1–13. https://doi.org/10.3389/fmicb.2019.00223
European Food Safety Authority (2010) Scientific opinion on dietary reference values for varbohydrates and dietary fibre. EFSA J 8:1–77. https://doi.org/10.2903/j.efsa.2010.1462
Institute of Medicine (2011) Dietary reference intakes for calcium and vitamin D. The National Academies Press, Washington DC
Schieber A, Stintzing FC, Carle R (2001) By-produtcs of plant food processing as a source of functional compounds—recent developments. Trends Food Sci Technol 12:401–413. https://doi.org/10.1016/S0924-2244(02)00012-2
Elleuch M, Bedigian D, Roiseux O et al (2011) Dietary fibre and fibre-rich by-products of food processing: characterisation, technological functionality and commercial applications: a review. Food Chem 124:411–421. https://doi.org/10.1016/j.foodchem.2010.06.077
Sharma SK, Bansal S, Mangal M et al (2016) Utilization of food processing by-products as dietary, functional, and novel fiber: a review. Crit Rev Food Sci Nutr 56:1647–1661. https://doi.org/10.1080/10408398.2013.794327
Schmid V, Trabert A, Schäfer J et al (2020) Modification of apple pomace by extrusion processing: studies on the composition, polymer structures, and functional properties. Foods 9:1–25. https://doi.org/10.3390/foods9101385
Schmid V, Trabert A, Keller J et al (2021) Functionalization of enzymatically treated apple pomace from juice production by extrusion processing. Foods 10:1–21. https://doi.org/10.3390/foods10030485
Schmid V, Steck J, Mayer-Miebach E et al (2021) Extrusion processing of pure chokeberry (Aronia melanocarpa) pomace: impact on dietary fiber profile and bioactive compounds. Foods 10:1–19. https://doi.org/10.3390/foods10030518
Houben K, Jolie RP, Fraeye I et al (2011) Comparative study of the cell wall composition of broccoli, carrot, and tomato: structural characterization of the extractable pectins and hemicelluloses. Carbohydr Res 346:1105–1111. https://doi.org/10.1016/j.carres.2011.04.014
Serena A, Knudsen KB (2007) Chemical and physicochemical characterisation of co-products from the vegetable food and agro industries. Anim Feed Sci Technol 139:109–124. https://doi.org/10.1016/j.anifeedsci.2006.12.003
Ring SG, Selvendran RR (1978) Purification and methylation analysis of cell wall material from Solanum tuberosum. Phytochemistry 17:745–752. https://doi.org/10.1016/S0031-9422(00)94219-5
Kaisangsri N, Kowalski RJ, Wijesekara I et al (2016) Carrot pomace enhances the expansion and nutritional quality of corn starch extrudates. LWT 68:391–399. https://doi.org/10.1016/j.lwt.2015.12.016
Dar AH, Sharma HK, Kumar N (2014) Effect of extrusion temperature on the microstructure, textural and functional attributes of carrot pomace-based extrudates. J Food Process Preserv 38:212–222. https://doi.org/10.1111/j.1745-4549.2012.00767.x
Schmid V, Trabert A, Keller J et al (2021) Defined shear and heat treatment of apple pomace: impact on dietary fiber structures and functional properties. Eur Food Res Technol 247:2109–2122. https://doi.org/10.1007/s00217-021-03776-0
Urbat F, Müller P, Hildebrand A et al (2019) Comparison and optimization of different protein nitrogen quantitation and residual protein characterization methods in dietary fiber preparations. Front Nutr 6:127. https://doi.org/10.3389/fnut.2019.00127
Prosky L, Asp NG, Furda I et al (1985) Determination of total dietary fiber in foods and food products: collaborative study. J AOAC 68:677–679
McCleary BV, deVries JW, Rader JI et al (2010) Determination of total dietary fiber (CODEX definition) by enzymatic-gravimetric method and liquid chromatography: collaborative study. J AOAC 1:221–233
Bunzel M, Ralph J, Marita JM et al (2001) Diferulates as structural components in soluble and insoluble cereal dietary fibre. J Sci Food Agric 81:653–660. https://doi.org/10.1002/jsfa.861
Saeman JF, Bubl JL, Harris EE (1954) Quantitative saccharification of wood and cellulose. Ind Eng Chem. Anal Ed 1:35–37. https://doi.org/10.1021/i560137a008
De Ruiter GA, de, Schols HA, Voragen AGJ, et al (1992) Carbohydrate analysis of water-soluble uronic acid-containing polysaccharides with high-performance anion-exchange chromatography using methanolysis combined with TFA hydrolysis is superior to four other methods. Anal Biochem 1:176–185. https://doi.org/10.1016/0003-2697(92)90520-H
Gniechwitz D, Reichardt N, Blaut M et al (2007) Dietary fiber from coffee beverage: degradation by human fecal microbiota. J Agric Food Chem 55:6989–6996. https://doi.org/10.1021/jf070646b
Sweet DP, Shapiro RH, Albersheim P (1975) Quantitative analysis by various G.L.C. response-factor theories for partially methylated and partially ethylated alditol acetates. Carbohydr Res 40:217–225. https://doi.org/10.1016/S0008-6215(00)82604-X
Wefers D, Bunzel M (2016) Arabinan and galactan oligosaccharide profiling by high-performance anion-exchange chromatography with pulsed amperometric detection (HPAEC-PAD). J Agric Food Chem 64:4656–4664. https://doi.org/10.1021/acs.jafc.6b01121
Blumenkrantz N, Asboe-Hansen G (1973) New method for quantitative determination of uronic acids. Anal Biochem 54:484–489. https://doi.org/10.1016/0003-2697(73)90377-1
Müller-Maatsch J, Caligiani A, Tedeschi T et al (2014) Simple and validated quantitative 1H NMR method for the determination of methylation, acetylation, and feruloylation degree of pectin. J Agric Food Chem 62:9081–9087. https://doi.org/10.1021/jf502679s
Lee HS, Rouseff RL, Nagy S (1986) HPLC determination of furfural and 5-hydroxymethylfurfural in citrus juices. J Food Sci 51:1075–1076. https://doi.org/10.1111/j.1365-2621.1986.tb11239.x
Wibowo S, Grauwet T, Santiago JS et al (2015) Quality changes of pasteurised orange juice during storage: a kinetic study of specific parameters and their relation to colour instability. Food Chem 187:140–151. https://doi.org/10.1016/j.foodchem.2015.03.131
Abdel-Aal E-SM, Young JC, Rabalski I et al (2007) Identification and quantification of seed carotenoids in selected wheat species. J Agric Food Chem 55:787–794. https://doi.org/10.1021/jf062764p
Craft NE, Soares JH (1992) Relative solubility, stability, and absorptivity of lutein and β-carotene in organic solvents. J Agric Food Chem 40:431–434. https://doi.org/10.1021/jf00015a013
Zechmeister L, Polgár A (1943) Cis-trans isomerization and spectral characteristics of carotenoids and some related compounds. J Am Chem Soc 65:1522–1528
Abbel DC, Sporns P (1996) Rapid quantitation of potato glycoalkaloids by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. J Agric Food Chem 44(8):2292–2296
Nielsen SD, Schmidt JM, Kristiansen GH et al (2020) Liquid chromatography mass spectrometry quantification of α-solanine, α-chaconine, and solanidine in potato protein isolates. Foods 9:1–13. https://doi.org/10.3390/foods9040416
Ceymann M, Arrigoni E, Schärer H et al (2011) Rapid high performance screening method using UHPLC-MS to quantify 12 polyphenol compounds in fresh apples. Anal Methods 3:1774–1778. https://doi.org/10.1039/c1ay05152k
Malec M, Le Quéré J-M, Sotin H et al (2014) Polyphenol profiling of a red-fleshed apple cultivar and evaluation of the color extractability and stability in the juice. J Agric Food Chem 62:6944–6954. https://doi.org/10.1021/jf500336v
Anderson RA (1982) Water absorption and solubility and amylograph characteristics on roll-cooked small grain products. Ceral Chem 59:265–269
Hernández-Ortega M, Kissangou G, Necoechea-Mondragón H et al (2013) Microwave dried carrot pomace as a source of fiber and carotenoids. FNS 4:1037–1046. https://doi.org/10.4236/fns.2013.410135
Kırbaş Z, Kumcuoglu S, Tavman S (2019) Effects of apple, orange and carrot pomace powders on gluten-free batter rheology and cake properties. J Food Sci Technol 56:914–926. https://doi.org/10.1007/s13197-018-03554-z
Hwang J-K, Choi J-S, Kim C-J et al (1998) Solubilization of apple pomace by extusion. J Food Process Preserv 22:477–491. https://doi.org/10.1111/j.1745-4549.1998.tb00364.x
Broxterman SE, Picouet P, Schols HA (2017) Acetylated pectins in raw and heat processed carrots. Carbohydr Polym 177:58–66. https://doi.org/10.1016/j.carbpol.2017.08.118
Steck J, Kaufhold L, Bunzel M (2021) Structural profiling of xyloglucans from food plants by high-performance anion-exchange chromatography with parallel pulsed amperometric and mass spectrometric detection. J Agric Food Chem 69:8838–8849. https://doi.org/10.1021/acs.jafc.1c02967
Wefers D, Tyl CE, Bunzel M (2014) Novel arabinan and galactan oligosaccharides from dicotyledonous plants. Front Chem 2:100. https://doi.org/10.3389/fchem.2014.00100
Mercier C (1977) Effect of extrusion-cooking on potato starch using a twin screw french extruder. Starch/Stärke 29:48–52. https://doi.org/10.1002/star.19770290204
Lai LS, Kokini JL (1991) Physicochemical changes and rheological properties of starch during extrusion (a review). Biotechnol Prog 7:251–266. https://doi.org/10.1021/bp00009a009
Schmid V, Steck J, Mayer-Miebach E et al (2020) Impact of defined thermomechanical treatment on the structure and content of dietary fiber and the stability and bioaccessibility of polyphenols of chokeberry (Aronia melanocarpa) pomace. Food Res Int 134:1–11. https://doi.org/10.1016/j.foodres.2020.109232
Beitane I, Krumina-Zemture G, Sabovics M (2018) Effect of germination and extrusion on the phenolic content and antioxidant activity of raw buckwheat (Fagopyrum esculentum Moench). Agron Res. https://doi.org/10.15159/ar.18.005
Bushway RJ, Ponnampalam R (1981) α-chaconine and α-solanine content of potato products and their stability during several modes of cooking. J Agric Food Chem 29:814–817
Lachman J, Hamouz K, Musilová J et al (2013) Effect of peeling and three cooking methods on the content of selected phytochemicals in potato tubers with various colour of flesh. Food Chem 138:1189–1197. https://doi.org/10.1016/j.foodchem.2012.11.114
Friedman M, Roitman JN, Kozukue N (2003) Glycoalkaloid and calystegine contents of eight potato cultivars. J Agric Food Chem 51:2964–2973. https://doi.org/10.1021/jf021146f
Machado RM, Toledo MCF, Garcia LC (2007) Effect of light and temperature on the formation of glycoalkaloids in potato tubers. Food Control 18:503–508. https://doi.org/10.1016/j.foodcont.2005.12.008
The German Federal Institute for Risk Assessment (BfR). Solanine in potatoes: green and strongly germinating potato tubers should be sorted out. BfR Opinion No 010/2018 of 23 April 2018. 2018.
Commisson Regulation (EU) 2017/2158 of 20 November 2017 Establishing mitigation measures and benchmark levels for the reduction of the presence of acrylamide in food. L 304/24.
Acknowledgements
The authors would like to express their thanks to Hannah Gaul, Jan Steffan, and Andrea Butterbrodt for supporting the sample preparation and analyses. Further, the authors thanks ThermoFisher Scientific for providing the extruder Process11 including all the needed equipment as well as GNT Europe GmbH for supplying the carrot pomace.
Funding
Open Access funding enabled and organized by Projekt DEAL. The IGF research project (20518 N) of the FEI was supported via AiF within the program for promoting the Industrial Collective Research (IGF) of the German Federal Ministry for Economic Affairs and Climate Action (BMWK), based on a resolution of the German Parliament.
Author information
Authors and Affiliations
Contributions
AT: Data curation, investigation, methodology, visualization, writing—original draft, review and editing original draft. VS: Data curation, investigation, methodology, project administration, visualization, writing—original draft. JK: Project administration, methodology, supervision, writing—review and editing. MAE: Conceptualization, funding acquisition, project administration, resources, supervision, writing—review and editing. MB: Conceptualization, funding acquisition, project administration, resources, supervision, writing—review and editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human or animal subjects.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Trabert, A., Schmid, V., Keller, J. et al. Chemical composition and technofunctional properties of carrot (Daucus carota L.) pomace and potato (Solanum tuberosum L.) pulp as affected by thermomechanical treatment. Eur Food Res Technol 248, 2451–2470 (2022). https://doi.org/10.1007/s00217-022-04060-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-022-04060-5