Abstract
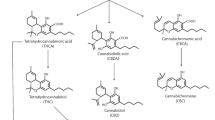
The increasing interest in cannabinoids, both in hemp plant material and hemp-derived products, has sparked a renewed interest in cannabinoid analysis, mostly by liquid chromatography. A simple isocratic HPLC method for analysing cannabinoids in hemp (Cannabis sativa and Cannabis indica) plant material and its extracts has been developed. It was demonstrated that separation of chromatographically critical cannabinoids can be successfully done by a careful selection of a few parameters like common mobile phase modifiers and column temperature. Column temperature proved to be very critical, even under isocratic elution. Analyses are performed within 8.5 min. The use of 275 nm detection wavelength provided about an order of magnitude better sensitivity compared to the established 228 nm wavelength normally used in cannabinoid analysis. The method was validated for 11 major cannabinoids present in Cannabis samples and products, namely: cannabidivarine, cannabidiolic acid, cannabigerolic acid, cannabigerol, cannabidiol, tetrahydrocannabivarin, cannabinol, Δ9-tetrahydrocannabinol, Δ8-tetrahydrocannabinol, tetrahydrocannabinolic acid, and cannabichromene. The assessed limits of detection and the limits of quantitation range from 7 to 205 ng/mL and from 23 to 684 ng/mL, respectively. Being isocratic, with minimum adaptation, the method can be applied for screening work using a shorter column or for a better performing analysis by employing a longer column.


Similar content being viewed by others
References
Gambaro VE, Froldi R, Saligari E, Dell’Acqua L, Salomone E, Roses OE (1995) Acta Toxicol Argent 3:11–13
Gibson CR, Williams RD, Browder RO (1998) J Anal Toxicol 22:179
Ross SA, Mehmedic Z, Murphy TP, Elsohly MA (2000) J Anal Toxicol 24:715–717
Raharjo TJ, Verpoorte R (2004) Phytochem Anal 15:79–94
Pellegrini M, Marchei E, Pacifici R, Pichini S (2005) J Pharm Biomed Anal 36:939–946
Gambaro V, Dell’Acqua L, Fare F, Froldi R, Saligari E, Tassoni G (2002) Anal Chim Acta 468:245–254
Cardenia V, Toschi TG, Scappini S, Rubino RC, Rodriguez-Estrada MT (2018) J Food Drug Anal 26:1283–1292
United Nations Office on Drugs and Crime (2009) Recommended methods for the identification and analysis of cannabis and cannabis products. United Nations Publications, New York
Wang M, Wang Y-H, Avula B, Radwan MM, Wanas AS, van Antwerp J, Parcher JF, ElSohly MA, Khan IA (2016) Cannabis Cannabinoid Res 1:262–271
Dussy FE, Hamberg C, Luginbühl M, Schwerzmann T, Briellmann TA (2005) Forensic Sci Int 149:3–10
Kemp PM, Abukhalaf IK, Manno JE, Manno BR, Alford DD, Abusada GA (1995) J Anal Toxicol 19:285–291
Steinmeyer S, Bregel D, Warth S, Kraemer T, Moeller MR (2002) J Chromatogr B 772:239–248
Leghissa A, Hildenbrand ZL, Foss FW Jr, Schug KA (2017) J Sep Sci 41:459–468
Pacifico D, Miselli F, Carboni A, Moschella A, Mandolino G (2007) Euphytica 160:231–240
Zuk-Golaszewska K, Golaszewski J (2018) J Elem 23:971–984
Burstein SH (2014) Bioorg Med Chem 22:2830–2843
Maurya N, Velmurugan BK (2018) Chem Biol Interact 293:77–88
Izzo AA, Capasso R, Aviello G, Borrelli F, Romano B, Piscitelli F, Gallo L, Capasso F, Orlando P, Di Marzo V (2012) Br J Pharmacol 166:1444–1460
Brierley DI, Samuels J, Duncan M, Whalley BJ, Williams CM (2016) Psychopharmacology 233:243–254
Velasco G, Hernández-Tiedra S, Dávila D, Lorente M (2016) Prog Neuro-Psychopharmacol Biol Psychiatry 64:259–266
Takeda S, Okajima S, Miyoshi H, Yoshida K, Okamoto Y, Okada T, Amamoto T, Watanabe K, Omiecinski CJ, Aramaki H (2012) Toxicol Lett 214:314–319
De Backer B, Debrus B, Lebrun P, Theunis L, Dubois N, Decock L, Verstraete A, Hubert P, Charlier C (2009) J Chromatogr B 877:4115–4124
Aizpurua-Olaizola O, Omar J, Navarro P, Olivares M, Etxebarria N, Usobiaga A (2014) Anal Bioanal Chem 406:7549–7560
Zgair A, Wong JCM, Sabri A, Fischer PM, Barrett DA, Constantinescu CS, Gershkovich P (2015) J Pharm Biomed Anal 114:145–151
Gul W, Gul SW, Radwan MM, Wanas AS, Mehmedic Z, Khan II, Sharaf MH, ElSohly MA (2015) J AOAC Int 98:1523–1528
Giese MW, Lewis MA, Giese L, Smith KM (2015) J AOAC Int 98:1503–1522
Citti C, Ciccarella G, Braghiroli D, Parenti C, Vandelli MA, Cannazza G (2016) J Pharm Biomed Anal 128:201–209
Patel B, Wene D, Fan Z (2017) J Pharm Biomed Anal 146:15–23
Ciolino LA, Ranieri TL, Taylor AM (2018) Forensic Sci Int 298:438–447
Mudge EM, Murch SJ, Brown PN (2017) Anal Bioanal Chem 409:3153–3163
Cannabinoids for potency testing in two methods with alternate elution orders by LC-UV. https://www.phenomenex.com/ViewDocument/?id=12+cannabinoids+for+potency+testing+in+two+methods+with+alternate+elution+orders+by+lc-uv+(tn-1225). Accessed 21 Jan 2019
Cannabinoids on raptor ARC-18 1.8 μm by LC-UV. https://www.restek.com/chromatogram/view/LC_GN0579/ARC-18. Accessed 21 Jan 2019
Qualitative and quantitative determination of cannabinoid profiles and potency in CBD hemp oil using LC/UV and mass selective detection. https://www.agilent.com/cs/library/applications/5991-8313EN_Cannabis_AppNote.pdf. Accessed 21 Jan 2019
Cannabinoids by LC-MS/MS using Kinetex 2.6 μm Biphenyl LC Column. https://www.phenomenex.com/ViewDocument/?id=10+cannabinoids+by+lc-ms_ms+using+kinetex+2.6+%u03bcm+biphenyl+lc+column. Accessed 21 Jan 2019
Eight cannabinoids by HPLC-UV. https://www.conflabs.com/wp-content/uploads/2018/03/EIght-Cannabinoids-by-HPLC-UV-v1.1.pdf. Accessed 21 Jan 2019
Separation of 12 natural cannabinoids using a Kinetex® Polar C18 column under methanol and acetonitrile conditions. https://www.phenomenex.com/ViewDocument/?id=separation+of+12+natural+cannabinoids+using+a+kinetex+polar+c18+column+under+methanol+and+acetonitrile+conditions. Accessed 21 Jan 2019
Neue UD (1997) In: Neue UD (ed) HPLC columns: theory, technology and practice. Wiley-VCH, New York, pp 351–379
Dolan JW (2002) J Chromatogr A 965:195–205
Analysis of biologically active compounds: cannabinoids. http://www.uct-uit-cooperation.eu/docs/stranska_rafa_interactive_seminar.pdf. Accessed 25 Jan 2019
Hazekamp A, Peltenburg A, Verpoorte R, Giroud C (2005) J Liq Chromatogr R T 28:2361–2382
Ramsperger HC, Porter CW (1926) J Am Chem Soc 48:1267–1273
Hillig KW, Mahlberg PG (2004) Am J Bot 91:966-975
Acknowledgement
The author acknowledges the financial support from the Slovenian Research Agency (research core funding No. P1-0005).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares that he has no conflict of interest.
Compliance with ethics requirements
This article does not contain any studies with human or animal subjects.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This manuscript is based on a contribution given at CHIMALI 2018, Italian Food Chemistry Congress, Camerino, September 24–27, 2018.
Rights and permissions
About this article
Cite this article
Križman, M. A simplified approach for isocratic HPLC analysis of cannabinoids by fine tuning chromatographic selectivity. Eur Food Res Technol 246, 315–322 (2020). https://doi.org/10.1007/s00217-019-03344-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-019-03344-7