Abstract
Most previous studies have been focused on the variation of tea chemical composition by fermentative processes as well as different cultivars and regions. The detailed changes of flavonoid profiles were described for the first time by each processing step of green and black tea leaves in this study. A total of 24 flavonoid derivatives including catechins, theaflavins, and flavonols were separated and identified from the tea samples based on UPLC-DAD-QToF/MS data and constructed library. Among these, the fragmentation pathway of theaflavins was proposed specifically in positive ionization mode for structural interpretation. During leaf processing, the individual flavonols were changed as diverse patterns according to their aglycone types and glycosylated forms, but their total content showed a slight difference. EGCG and ECG were increased after roasting approximately twofold higher than that of fresh leaves (EGCG, 2709.5 →6085.6; ECG, 1548.0 →2318.2 mg/100 g dry weight, respectively) in green tea while considerably decreased their contents due to oxidation and conversion to theaflavins after fermentation during black tea processing. Especially, the drying steps also found to be factor to influence positively to increase the flavonoid contents in both tea processing. Therefore, this result indicated that detailed conditions of each processing step played important roles in changing the flavonoid profiles from tea leaves.
Graphical abstract

Similar content being viewed by others
Introduction
Green tea (Camellia sinensis) is one of the most widely consumed beverages in mainly Asia and Europe. Health-promoting compounds such as catechins, theaflavins, thearubigins, flavonols, caffeine, and other phenolic compounds are predominantly distributed in the leaves of green and black (fermented) tea [1,2,3,4]. Catechin derivatives are belonging to flavan-3-ols and known to be abundant in tea products [5]. Especially, theaflavins are the responsible compounds to determine the color and taste of black tea infusions as conjugates of epicatechin (EC), epigallocatechin (EGC), and gallic acid by enzymatic oxidation reaction in fermentations [6].
Biological activities have been evaluated variously from tea products for potential health benefit. Epicatechin 3-O-gallate (ECG) and epigallocatechin 3-O-gallate (EGCG) were reported as the major compounds with strong radical-scavenging activities [7]. In addition, myricetin 3-O-galactoside and myricetin 3-O-glucoside showed potent antioxidant activities [8]. In tea consumption, dietary polyphenols played an important role in the preventive aspects of human diseases such as Alzheimer’s, cardiovascular disease, diabetes mellitus, and obesity [9,10,11,12], and revealed remarkable activity as anti-carcinogenic agent [13,14,15].
Most polyphenolic studies have performed as qualitative and quantitative analysis using liquid chromatography–mass spectrometric (LC–MS) technologies. In addition, polyphenolic composition was focused on observation at the level of fermentation from green, oolong, black, and white teas [4, 16]. A total of 40 flavonoids were tentatively identified including flavonol glycosides with glucose, galactose, rhamnose, and rutinose from green and black teas using a triple quadrupole MS [17]. Similarly, Lin et al. [18] determined a total of 63 flavonoids from various fermented tea samples, but other compounds except for two acylated flavonol glycosides (kaempferol 3-O-6′′-p-coumaroylglucoside and kaempferol 3-O-2′′,6′′-di-p-coumaroylglucoside) were still unclear in acylated positions.
The phytochemical composition of tea leaves is affected by several factors such as cultivated regions, climatic conditions, varieties, brewing techniques, and processing conditions. The profiles of catechin derivatives including EC, EGC and EGCG were differentiated by geographical origins and climatic conditions in green teas [19, 20]. In brewing time of green teas, the content of EC, EGC and EGCG were no longer increased after 4 to 5 minutes [21]. Furthermore, Peterson et al. [22] suggested optimal brewing techniques (tea weight and brewing time) as well as varietal differences for determining the contents of catechins, theaflavins, and thearubigins in black teas. In leaf processing step, a slight increase of total catechins and polyphenols was observed during withering stage of Assam (C. sinensis var. assamica) green and black teas [23]. During black tea preparation, the conversion of phenolic compounds was derived by different fermentation and drying stages with much higher loss of catechins [24]. Far-infrared radiation (FIR) replacing the roasting step might also enhance total catechins and phenols [25]. These results indicated that processing methods might be responsible for tea quality related with contents of catechin derivatives.
Despite conducting qualitative and quantitative analysis of phenolic compounds on tea leaves and products, the accurate determination of flavonoid derivatives at each step of tea leaf processing remains unstudied. In this study, a comprehensive flavonoid library was constructed from the literature data, and was used for the identification of individual flavonoid component with UPLC-DAD-QToF/MS analysis. Therefore, a quantitative change was evaluated at each steps of leaf processing in green and black teas based on flavonoid profiles. Furthermore, it will be valuable to provide chemical information for optimal processing conditions to produce high quality teas.
Materials and methods
Chemical reagents
A galangin was used as an internal standard (Extrasynthese, Genay, France). Acetonitrile, methanol (MeOH), and purified water were purchased from Fisher Scientific (Fair Lawn, NJ, USA). Formic acid was obtained from the Junsei Chemical Co. Ltd. (Tokyo, Japan).
Plant materials
Fresh tea leaves (variety Chamnok) were harvested from same age of trees (fifteen years old) in May 2014 for green tea and September 2014 for black tea. These tea trees were cultivated from Tea Industry Institute at Boseong-gun, Jeollanamdo, Republic of Korea (latitude, 34°46′N; longitude, 127°46′E) by organic farming system.
Leaf processing methods of green and black teas
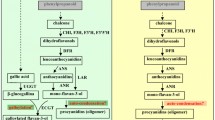
The scheme of leaf processing steps is presented in Fig. 1. For preparing green tea, the fresh leaves were taken and roasted at 250–300 °C for 10 min (roasting) to inactivate enzymatic activities and followed by rolling of roasted leaves meanwhile allowing it to cool for 10 min (rolling). To generate the flavor, the rolled leaves were further dried on oven: first at 150–200 °C for 10 min (first drying), then at 100–150 °C for 10 min (second drying), and finally at 90–100 °C for 10 min (third drying) under hot air. For preparing black tea, the fresh leaves were withered for 24 h at room temperature to reduce the moisture content of leaves below 60% (withering). The leaves were rolled for 30 min (rolling) and then allowed to ferment naturally at room temperature for 3 h (fermentation). After fermentation, two steps of drying were conducted; at 110 °C for 20 min and 90–100 °C for 10 min (first drying and second drying), respectively. After each processing stage completed, all samples were lyophilized (Programmable Freeze Dryer, Ilshin Lab Co. Ltd., Republic of Korea), pulverized, and stored below − 60 °C.
Extraction of catechins, theaflavins, and flavonols
Extraction procedures of flavonoids were conducted according to the method described by Kim et al. [26] with slight modification. The powdered samples (0.2 g) were shaken for 5 min with 40 mL of MeOH:water:formic acid (50:45:5, v/v/v) containing 20 µg/mL of internal standard (galangin). After centrifugation (3000 rpm, 4 °C, 15 min), the supernatant was immediately filtered using a syringe filter (PVDF 0.20 µm, Whatman, Kent, England), and then 0.5 mL of the filtrate was diluted with water to 5 mL of final volume. The extract was semi-purified using the Sep-pak C18 classic cartridge (Waters Co., Milford, MA, USA). The cartridge was activated by 2 mL of MeOH, followed by 2 mL of water for conditioning. The diluted extract was loaded into the cartridge, and impurities were removed by washing with 2 mL of water. The crude flavonoid extract was finally eluted by 3 mL of MeOH and concentrated using N2 gas, and then re-dissolved with 0.5 mL of MeOH:water:formic acid (50:45:5, v/v/v) prior to UPLC-DAD-QToF/MS analysis.
UPLC-DAD-QToF/MS analysis
The individual flavonoid components were analyzed using an ultra-performance liquid chromatography-diode array detector (Waters Co., Milford, MA, USA) and quadrupole time of flight mass spectrometry (Waters Micromass, Manchester, UK) (UPLC-DAD-QToF/MS) equipped with Kinetex 1.7 µm XB C18 100A column (150 × 2.1 mm i.d., Phenomenex, Torrance, CA, USA). According to our previous report [27], chromatographic condition was conducted: flow rate (0.3 mL/min), column oven temperature (30 °C), and representative wavelengths (280 nm for catechins and theaflavins; 350 nm for flavonols). The mobile phase was 0.5% formic acid in water (A) and 0.5% formic acid in acetonitrile (B). Elution gradient used as follows: initial 5% B; 20 min, 25% B; 25 min, 50% B; 30–32 min, 90%, and 35–40 min, 5% B. The mass spectrometric settings used was: capillary voltage 3.5 kV, sampling cone voltage 40 V, source temperature 120 °C, desolvation temperature 500 °C, and desolvation gas 1050 L/h. Mass analysis was run in positive ionization mode using an electrospray ionization (ESI) source, and their range measured at 200–1200 m/z in full-scan mode. The quantification was performed using an internal standard without considering relative response factor. All experimental analyses were conducted in triplicates.
Construction of LC–MS library for flavonoid identification
The flavonoid library was constructed from 21 literature sources related to green and black tea leaves based on structural evidences elucidated by NMR spectroscopy and mass spectrometry, and contained positive- and negative-ion fragmentations (Table S1).
Statistical analysis
To compare between treatments, a significant difference was verified by one-way ANOVA with Duncan’s multiple range test (p < 0.05) in SPSS (version 24.0, SPSS Inc., Chicago, IL).
Results and discussion
Identification of catechins, theaflavins, and flavonols in green and black tea samples
From leaves of green and black teas, the mass fragmentations of flavonoid derivatives were summarized and presented in the constructed LC–MS library for identification based on the literatures (Table S1). The library contains a total 64 flavonoids including catechins, theaflavins, flavonols, and flavones, and provides their positive- and negative-ion fragmentations. The positive fragmentation was composed of both reported and proposed product ions.
On the previous studies, phenolic compounds such as flavonoids, hydroxycinnamic acid derivatives, and tannins have been characterized majorly by negative ionization mode [28, 29]. However, the present positive ionization study of flavonoids provides sodium (Na+, m/z 23) and potassium (K+, m/z 39) adduct ions that can help to distinct the parent ion easily, when peaks presented at low concentration as well as complexly, compared to negative-ion fragmentations (Table S1; Table 1). Actually, positive ions including [M + Na]+, [M + H]+, and glycosidic loss were fragmented clearly from flavonoid derivatives of green and black tea samples by Atoui et al. [30].
A total of 24 flavonoid derivatives including catechins (4), theaflavins (4), and flavonols (16) were separated and identified from the green and black tea samples using UPLC-DAD-QToF/MS (Table 1). Their chemical structures were described by R groups of each class in Fig. 2. The peak identification was completed by comparing retention time, UV spectra, and mass fragmentation presented in the literatures of constructed library.
(−)-epicatechin 3-O-gallate (ECG) and (−)-epigallocatechin 3-O-gallate (EGCG) were known as major compound in tea samples. Nevertheless, their positive-ion fragmentations showed the limitation that reported only parent ions of m/z 443 and 459 [M + H]+ from green and white tea samples, respectively [31]. EGCG (peak 3) was additionally observed for m/z 307 [M + H-Gall]+ and 289 [M + H-Gall-H2O]+ in addition to m/z 481 and 459 corresponding to [M + Na]+ and [M + H]+. The fragments of ECG (peak 10) showed similar pattern to EGCG, and yielded regular ions at m/z 465, 443, 291, and 273. Unlike EGCG and ECG, potassium adduct ions were distinctively detected in aglycone types such as (−)-epigallocatechin (EGC, peak 1) and (−)-epicatechin (EC, peak 2). These compounds were fragmented with loss of H2O as well as adduct ions (Na+ and H+) from parent ions (Table 1).
Only the positive ions corresponding to [M + K]+, [M + Na]+, and [M + H]+ were presented in theaflavins isolated from fermented tea samples [3, 18]. However, in Fig. 3b,c and Table 1, the detailed fragmentations of theaflavins were proposed based on the structural pattern presented in negative ionization mode by Yassin et al. [32]. The fragments of theaflavins were produced primarily through Retro-Diels–Alder (RDA) fission (C7H6O3, 138 Da) with the loss of H2O and galloyl moiety (Gall, 152 Da). The main product ions of theaflavin 3-O-gallate (peak 21) and theaflavin 3′-O-gallate (peak 22) were largely divided into non-applied group (m/z 739 [M + Na]+, 717 [M + H]+, 699 [M + H-H2O]+, 547 [M + H-Gall-H2O]+, and 529 [M + H-Gall-2H2O]+), and applied group (m/z 579 [M + H-C7H6O3]+, 561 [M + H-H2O-C7H6O3]+, 409 [M + H-Gall-H2O-C7H6O3]+ and 391 [M + H-Gall-2H2O-C7H6O3]+) relation to RDA fission (Fig. 3c). Especially, theaflavin 3,3′-di-O-gallate (peak 23) was determined as major theaflavin with fragmentation of m/z 891 [M + Na]+, 869 [M + H]+, 731 [M + H-C7H6O3]+, 717 [M + H-Gall]+, 699 [M + H-Gall-H2O]+, 579 [M + H-Gall-C7H6O3]+, 561 [M + H-Gall-H2O-C7H6O3]+, 547 [M + H-2Gall-H2O]+, 529 [M + H-2Gall-2H2O]+, 409 [M + H-2Gall-H2O-C7H6O3]+ and 391 [M + H-2Gall-2H2O-C7H6O3]+ (Fig. 3b). Thus, the fragmentations of theaflavin compounds were specifically described for the first time in positive ionization mode.
Mass spectrometric characteristics of representative flavonoids isolated from green and black teas (Camellia sinensis). a Kaempferol 3-O-(3′’’-O-galactosyl)rutinoside; b theaflavin 3,3′-di-O-gallate; c proposed fragmentation pathway of theaflavin 3-O-gallate. Abbreviations: Gal galactose; Gall gallic acid; RDA Retro-Diels–Alder; Rham rhamnose; Rut, rutinose
Flavonol-related derivatives were isolated from green and black tea samples, and found to be a class of kaempferol, quercetin, and myricetin conjugated with glycosides such as glucose, galactose, rutinose, galactosylrutinose, and glucosylrutinose (Fig. 2; Table 1). In the previous studies, de la Luz Cadiz-Gurrea et al. [33] who isolated four kaempferol glycosides from green tea extract did not provide the precise bonding position of glycosides. In addition, Scoparo et al. [17] could not confirm the glycosylated type and position as seen in myricetin 3-O-hexoside-rutinoside, quercetin 3-O-galactoside-rutinoside, quercetin 3-O-glucoside-rutinoside, kaempferol 3-O-galactoside-rutinoside, and kaempferol 3-O-glucoside-rutinoside isolated from green and black teas. On the current study, the isolated six flavonol tri-glycosides were characterized as galactosylrutinoside (peaks 4, 8, and 12) and glucosylrutinoside (peaks 5, 9, and 16) with the same molecular weight corresponding to kaempferol, quercetin, and myricetin, respectively (Fig. 2). Among them, it was confirmed that galactosyl form elutes faster than glucosyl form in the same aglycone (Fig. 4b) [34]. From the recent studies, these tri-glycosidic forms were elucidated as conjugates of galactose or glucose at 3′′′- position of the rutinose moiety using NMR spectroscopy [35, 36]. Therefore, kaempferol 3-O-(3′′′-O-galactosyl)rutinoside (peak 12) was identified as major flavonol observed at m/z 779 [M + Na]+ and 757 [M + H]+, and fragmented by losing of glycosidic bond clearly corresponding to 595 [M + H-Gal]+, 449 [M + H-Gal-Rham]+ and 287 [M + H-Gal-Rut]+ (Fig. 3a). The other tri-glycosides were also completely confirmed as kaempferol 3-O-(3′′-O-glucosyl)rutinoside (peak 16, m/z 757), quercetin 3-O-(3′′-O-galactosyl)rutinoside (peak 8, m/z 773), quercetin 3-O-(3′′-O-glucosyl)rutinoside (peak 9, m/z 773), myricetin 3-O-(3′′-O-galactosyl)rutinoside (peak 4, m/z 789), and myricetin 3-O-(3′′′-O-glucosyl)rutinoside (peak 5, m/z 789) in [M + H]+ ions (Table 1).
Changes of flavonoid compositions during leaf processing steps
In each processing step of the green and black teas, the changes of composition and content were showed differently by catechins, theaflavins, and flavonols (Table 1). The final green and black teas contained the total catechins (mg/100 g DW) of 8857.2 and 1803.0, respectively, which are similar with data (7 green teas, range 5150–8430 → mean 6700; 12 black teas, range 560–4750 → mean 1540) provided by Khokhar and Magnusdottir [37] as well as a report (Meifoo and Shanghai green teas, 11,250 and 10,045; Fujian black, 1531) of Zuo et al. [4]. In green tea, total flavonoid content was dramatically increased after roasting step. Especially, as major catechins, EGCG and ECG were reaching to the highest level on the final product, and their contents (mg/100 g DW) increased approximately twofold higher than that of fresh leaves (EGCG, 2709.5 → 6085.6; ECG, 1548.0 → 2318.2). Theaflavins were present in the fresh leaves but completely disappeared after roasting. Heating processing (250–300 °C) was one of the important factors that affect the chemical composition of tea, and considered to cause the increase or decrease of certain components through drying or hydrolysis under water-free conditions, while Kim et al. [38] reported that the catechin contents were decreased with increasing infused temperature in green tea liquor. However, since the flavonol content was slightly increased after roasting (1054.3 → 1203.4 mg/100 g DW), it was hard to interpret that the great increase of catechin was entirely affected by water evaporation of the fresh leaf. Based on these points, the roasting step would be also expected to minimize the loss of catechin content by inactivating the polyphenol oxidase (PPO) enzyme leading to catechin degradation in fresh leaf [23]. On the other hand, the contents of catechins and flavonols were significantly decreased by rolling step, and then increased again after drying steps in green tea. By Turkmen and Velioglu [39], as the rolling time was shortening, the loss of ECG and EGCG contents was reduced during leaf processing. The flavonols showed a tendency to reach maximum level through the three steps of drying, but there were no significant differences in mono- and tri-glycosides as well as total content until completion of the final product over second and third drying (Fig. 5b). In details, as major class, mono- and tri-glycosides of kaempferol (peak 12, 15, 16, and 18) were showed the highest contents, while mono- and tri-glycosides of quercetin (peak 8, 9, 13, and 14) were slightly lower than the previous step on the final product (Table 1). In green tea processing, roasting and drying were the most important factors in changing individual catechin and flavonol contents, and it was concluded that the flavonoid content could be optimized by controlling these steps in the future.
Fermentation during the production of black tea leaf is the important step that shows the greatest change in flavonoid composition and content. Most studies have been evaluated flavonoid changes in simple fermented differences by product types such as black, oolong, and Pu’er teas [18, 40]. Figure 4a chromatogram indicated that catechins were converted certainly to theaflavins through the fermentation step of black tea. In general, fermentation is one of the most affected methods to change the ingredients in agro-food processing. For example, as the major isoflavones of soybean, genistin and malonylgenistin tended to reproduce their hydrolyzed forms, namely genistein and acetylgenistin during fermentation [41]. In the contrary, catechins induces condensation reaction after oxidation by PPO enzyme to form theaflavins, thearubigins, and proanthocyanidins with large molecular weight during black tea processing, and the higher concentration of theaflavins might be caused by this chemical phenomenon (Fig. 4c) [6, 42]. In the present study, the catechin contents (mg/100 g DW) were slightly increased in the withering (4692.1) and then decreased significantly from rolling (2562.6) to fermentation (933.9) steps similar to the previous reports by Astill et al. [23] and Kim et al. [16]. The reduced content of individual catechins was increased approximately twofold again when it became the final product after drying (Table 1). The total theaflavin content (mg/100 g DW) increased remarkably throughout whole black tea processing following order: withering (105.7) → rolling (164.7) → fermentation (300.5) → first drying (486.4) → second drying (480.6) → final product (636.8). As shown in Figs. 4c and 5a, theaflavin 3,3′-di-O-gallate (305.6) and theaflavin 3-O-gallate (168.4) which were advanced with gallic acid in biosynthetic pathway, showed the greatest increases of 4.5- and sixfold as predominant compounds compared to fresh leaf, respectively. Unlike this proposed synthetic pattern, Liang et al. [43] reported that the concentration of theaflavin and theaflavin 3′-O-gallate was found to be approximately twofold higher than theaflavin 3-O-gallate and theaflavin 3,3′-di-O-gallate in black tea samples. Recently, it was provided that theaflavin-3,3′-di-O-gallate could be regulated inflammatory factors such as tumor necrosis factor-alpha (TNF-α), and interleukin (IL)-1β and -6 as potential therapeutic candidate [44].
On flavonol contents of black tea, significant differences were found between fresh leaf and final product, but only 23% of reduction was occurred in comparison with green tea. According to Del Rio et al. [45] study, an about 30% of reduction was discovered in total flavonols during black tea processing. Besides, Zhao et al. [31] investigated that the flavonol contents were not changed by different fermented degrees from green to Pu’er tea samples. Briefly, in Table 1, the change of kaempferol and quercetin glycosides had no significant difference between fresh leaf and final product. However, myricetin glycosides showed different patterns depending on the glycosidic forms. Interestingly, the tri-glycosides of myricetin were completely disappeared after fermentation as well as their mono-glycosides of final product have reduced more than 50% compared to fresh leaf. There is no report about focusing changes of flavonols during tea processing. It was considered that additional processing methods for increasing flavonol level are required to further optimize green and black tea qualities.
The composition of chemical compounds such as EGCG, ECG, and theaflavins could be evaluated as a potential quality indicator for grading of green and black tea infusion [46]. From our study, rolling step normally decreased catechins and flavonols both green and black tea. Thus, detailed rolling conditions are necessary to investigate minimum loss of catechins and flavonols. Theaflavins which were increased only in black tea processing might be considered to utilize as a health beneficial compounds for high qualities of black tea. However, the actual reaction mechanism of compounds on processing requires further studies. In the future, for the representative tea products by origins, it will be possible to evaluate the functional ingredients by their processing conditions to control the qualities more efficiently.
Conclusions
The previous studies have been focused on the optimization of tea quality by comparing different varieties and cultivated regions. This study described specifically the changes of individual flavonoids according to each processing step of green and black tea leaves. A total of 24 flavonoid derivatives including catechins, theaflavins, and flavonols were separated and identified from the tea samples based on UPLC-DAD-QToF/MS data and constructed library. Among these, the fragmentations pathway of theaflavins was proposed for the first time in positive ionization mode. The contents of catechins, theaflavins, and flavonols showed a similar tendency to that of the previous studies on the final tea products (green and black), respectively. During leaf processing, the individual flavonol contents were changed diversely according to their aglycone types and glycosylated forms. In particular, roasting step generally enhanced catechin content including EGCG and ECG in green tea, but catechins were considerably reduced due to their oxidation and conversion to theaflavins after the fermentation step in black tea. The drying multi-steps also found to be factor to influence positively to increase the flavonoids in tea processing. Therefore, this result indicated that individual flavonoids might be optimized by controlling the detailed conditions of each processing step to obtain the excellent quality on final tea products.
References
Bastos DH, Saldanha LA, Catharino RR, Sawaya ACHF, Cunha IBS, Carvalho PO, Eberlin MN (2007) Phenolic antioxidants identified by ESI-MS from yerba mate (Ilex paraguariensis) and green tea (Camelia sinensis) extracts. Molecules 12(3):423–432
Liu Y, Gao L, Liu L, Yang Q, Lu Z, Nie Z, Wang Y, Xia T (2012) Purification and characterization of a novel galloyltransferase involved in catechin galloylation in the tea plant (Camellia sinensis). J Biol Chem 287(53):44406–44417
Menet MC, Sang S, Yang CS, Ho CT, Rosen RT (2004) Analysis of theaflavins and thearubigins from black tea extract by MALDI-TOF mass spectrometry. J Agric Food Chem 52:2455–2461
Zuo Y, Chen H, Deng Y (2002) Simultaneous determination of catechins, caffeine and gallic acids in green, oolong, black and pu-erh teas using HPLC with a photodiode array detector. Talanta 57(2):307–316
Wang Y, Gao L, Shan Y, Liu Y, Tian YW, Xia T (2012) Influence of shade on flavonoid biosynthesis in tea (Camellia sinensis (L.) O. Kuntze). Sci Hortic 141:7–16
Dwyer JT, Peterson J (2013) Tea and flavonoids: where we are, where to go next. Am J Clin Nutr 98(6):1611S–1618S
Yang Z, Tu Y, Baldermann S, Dong F, Xu Y, Watanabe N (2009) Isolation and identification of compounds from the ethanolic extract of flowers of the tea (Camellia sinensis) plant and their contribution to the antioxidant capacity. Food Sci Technol 42(8):1439–1443
Manir MM, Kim JK, Lee BG, Moon SS (2012) Tea catechins and flavonoids from the leaves of Camellia sinensis inhibit yeast alcohol dehydrogenase. Bioorganic Med Chem 20(7):2376–2381
Kuriyama S, Shimazu T, Ohmori K, Kikuchi N, Nakaya N, Nishino Y, Tsubono Y, Tsuji I (2006) Green tea consumption and mortality due to cardiovascular disease, cancer, and all causes in Japan. J Am Med Assoc 296(10):1255–1265
Nagao T, Hase T, Tokimitsu I (2007) A green tea extract high in catechins reduces body fat and cardiovascular risks in humans. Obesity 15(6):1473–1483
Rezai-Zadeh K, Arendash GW, Hou H, Fernandeza F, Jensen M, Runfeldt M, Shytlea RD, Tan J (2008) Green tea epigallocatechin-3-gallate (EGCG) reduces β-amyloid mediated cognitive impairment and modulates tau pathology in Alzheimer transgenic mice. Brain Res 1214:177–187
Sabu MC, Smitha K, Kuttan R (2002) Anti-diabetic activity of green tea polyphenols and their role in reducing oxidative stress in experimental diabetes. J Ethnopharmacol 83(1):109–116
Azam S, Hadi N, Khan NU, Hadi SM (2004) Prooxidant property of green tea polyphenols epicatechin and epigallocatechin-3-gallate: implications for anticancer properties. Toxicol In Vitro 18(5):555–561
Chung LY, Cheung TC, Kong SK, Fung KP, Choy YM, Chan ZY, Kwok TT (2001) Induction of apoptosis by green tea catechins in human prostate cancer DU145 cells. Life Sci 68(10):1207–1214
Nakazato T, Ito K, Miyakawa Y, Kinjo K, Hozumi N, Ikeda T, Kizaki M (2005) Catechin, a green tea component, rapidly induces apoptosis of myeloid leukemic cells via modulation of reactive oxygen species production in vitro and inhibits tumor growth in vivo. Haematologica 90(3):317–325
Kim Y, Goodner KL, Park JD, Choi J, Talcott ST (2011) Changes in antioxidant phytochemicals and volatile composition of Camellia sinensis by oxidation during tea fermentation. Food Chem 129(4):1331–1342
Scoparo CT, de Souza LM, Dartora N, Sassaki GL, Gorin PAJ, Iacomini M (2012) Analysis of Camellia sinensis green and black teas via ultra high performance liquid chromatography assisted by liquid-liquid partition and two-dimensional liquid chromatography (size exclusion × reversed phase). J Chromatogr A 1222:29–37
Lin L, Chen P, Harnly JM (2008) New Phenolic components and chromatographic profiles of green and fermented teas. J Agric Food Chem 56:8130–8140
Kodama S, Ito Y, Nagase H, Yamashita T, Kemmei T, Yamamoto A, Hayakawa K (2007) Usefulness of catechins and caffeine profiles to determine growing area of green tea leaves of a single variety, Yabukita, in Japan. J Health Sci 53(4):491–495
Lee J, Lee B, Chung J, Hwang J, Lee S, Lee C, Hong Y (2010) Geographical and climatic dependencies of green tea (Camellia sinensis) metabolites: a 1H NMR-based metabolomics study. J Agric Food Chem 58(19):10582–10589
Shishikura Y, Khokhar S (2005) Factors affecting the levels of catechins and caffeine in tea beverage: estimated daily intakes and antioxidant activity. J Sci Food Agric 85:2125–2133
Peterson J, Dwyer J, Jacques P, Rand W, Prior R, Chui K (2004) Tea variety and brewing techniques influence flavonoid content of black tea. J Food Compos Anal 17:397–405
Astill C, Birch MR, Dacombe C, Humphrey PG, Martin PT (2001) Factors affecting the caffeine and polyphenol contents of black and green tea infusions. J Agric Food Chem 9:340–5347
Obanda M, Owuor PO, Mang’oka R (2001) Changes in the chemical and sensory quality parameters of black tea due to variations of fermentation time and temperature. Food Chem 75:395–404
Kim SY, Jeong SM, Jo SC, Lee SC (2006) Application of far-infrared irradiation in the manufacturing process of green tea. J Agric Food Chem 54(26):9943–9947
Kim HW, Kim JB, Cho SM, Chung MN, Lee YM, Chu SM, Che JH, Kim SN, Kim SY, Cho YS, Kim JH, Park HJ (2012) Anthocyanin changes in the Korean purple-fleshed sweet potato, Shinzami, as affected by steaming and baking. Food Chem 130:966–972
Lee M, Kim H, Kim YJ, Lee S, Jang H, Jung H, Kim S, Lee S, Choe J, Kim J (2016) Profiling of flavonoid glycosides in fruits and leaves of jujube (Zizyphus jujuba var. inermis (Bunge) Rehder) using UPLC-DAD-QTOF/MS. Korean J Food Preserv 23(7):1004–1011
Narváez-Cuenca C, Vincken J, Gruppen H (2012) Identification and quantification of (dihydro) hydroxycinnamic acids and their conjugates in potato by UHPLC-DAD-ESI-MSn. Food Chem 130:730–738
Simirgiotis MJ, Schmeda-Hirschmann G (2010) Determination of phenolic composition and antioxidant activity in fruits, rhizomes and leaves of the white strawberry (Fragaria chiloensis spp. chiloensis form chiloensis) using HPLC-DAD-ESI-MS and free radical quenching techniques. J Food Compos Anal 23:545–553
Atoui AK, Mansouri A, Boskou G, Kefalas P (2005) Tea and herbal infusions: their antioxidant activity and phenolic profile. Food Chem 89:27–36
Zhao Y, Chen P, Lin L, Harnly JM, Yu L, Li Z (2011) Tentative identification, quantitation, and principal component analysis of green pu-erh, green, and white teas using UPLC/DAD/MS. Food Chem 126(3):1269–1277
Yassin GH, Koek JH, Jayaraman S, Kuhnert N (2014) Identification of novel homologous series of polyhydroxylated theasinensins and theanaphthoquinones in the SII fraction of black tea thearubigins using ESI/HPLC tandem mass spectrometry. J Agric Food Chem 62:9848–9859
de la Luz Cadiz-Gurrea M, Fernandez-Arroyo S, Segura-Carretero A (2014) Pine bark and green tea concentrated extracts: antioxidant activity and comprehensive characterization of bioactive compounds by HPLC-ESI-QTOF-MS. Int J Mol Sci 15(11):20382–20402
Diaconeasa Z, Florica R, Rugina D, Lucian C, Socaciu C (2014) HPLC/PDA-ESI/MS identification of phenolic acids, flavonol glycosides and antioxidant potential in blueberry, blackberry, raspberries and cranberries. J Food Nutr Res 2(11):781–785
Hilal Y, Engelhardt UH (2009) A new myricetin-rhamnodiglucoside from Camellia sinensis. Nat Prod Res 23(17):1621–1629
Lakenbrink C, My Loc Lam T, Engelhardt UH, Wray V (2000) New flavonol triglycosides from tea (Camellia sinensis). Nat Prod Lett 14(4):233–238
Khokhar S, Magnusdottir SGM (2002) Total phenol, catechin, and caffeine contents of teas commonly consumed in the United Kingdom. J Agric Food Chem 50:565–570
Kim ES, Liang YR, Jin J, Sun QF, Lu JL, Du YY, Lin C (2007) Impact of heating on chemical compositions of green tea liquor. Food Chem 103(4):1263–1267
Turkmen N, Velioglu YS (2007) Determination of alkaloids and phenolic compounds in black tea processed by two different methods in different plucking seasons. J Sci Food Agric 87:1408–1416
Peterson J, Dwyer J, Bhagwat S, Haytowitz D, Holden J, Eldridgec AL, Beecherd G, Aladesanmi J (2005) Major flavonoids in dry tea. J Food Compos Anal 18(6):487–501
Lee MJ, Chung I, Kim H, Jung MY (2015) High resolution LC-ESI-TOF-mass spectrometry method for fast separation, identification, and quantification of 12 isoflavones in soybeans and soybean products. Food Chem 176:254–262
Sang S, Tian S, Meng X, Stark RE, Rosen RT, Yang CS, Ho C (2002) Theadibenzotropolone A, a new type pigment from enzymatic oxidation of (–)-epicatechin and (–)-epigallocatechin gallate and characterized from black tea using LC/MS/MS. Tetrahedron Lett 43:7129–7133
Liang Y, Lu J, Zhang L, Wu S, Wu Y (2003) Estimation of black tea quality by analysis of chemical composition and colour difference of tea infusions. Food Chem 80(2):283–290
Wu Y, Jin F, Wang Y, Li F, Wang L, Wang Q, Ren Z (2017) In vitro and in vivo anti-inflammatory effects of theaflavin-3,3′-digallate on lipopolysaccharide-induced inflammation. Eur J Pharmacol 794:52–60
Del Rio D, Stewart AJ, Mullen W, Burns J, Lean MEJ, Brighenti F, Crozier A (2004) HPLC-MSn analysis of phenolic compounds and purine alkaloids in green and black tea. J Agric Food Chem 52(10):2807–2815
Obanda M, Owuor PO (1997) Flavanol composition and caffeine content of green leaf as quality potential indicators of Kenyan black teas. J Sci Food Agric 74:209–215
Acknowledgements
The authors would like to acknowledge the research funding from the ‘Cooperative Research Program for Agriculture Science and Technology Development (Project No. PJ01178704)’ provided by the Rural Development Administration, Republic of Korea.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Compliance with ethics requirements
This article does not contain any studies with human or animal subject.
Electronic supplementary material
Below is the link to the electronic supplementary material.
217_2018_3201_MOESM1_ESM.docx
The LC–MS library based on literatures supposed to identify flavonoids in green and black teas (Camellia sinensis) (Supplemental Table 1) (DOCX 59 KB)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Lee, MK., Kim, HW., Lee, SH. et al. Characterization of catechins, theaflavins, and flavonols by leaf processing step in green and black teas (Camellia sinensis) using UPLC-DAD-QToF/MS. Eur Food Res Technol 245, 997–1010 (2019). https://doi.org/10.1007/s00217-018-3201-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-018-3201-6