Abstract
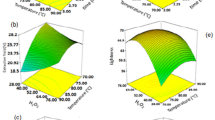
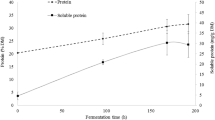
Brewers’ spent grain (BSG) and spent yeast (BSY) are the main by-products of the brewing industry, with currently limited valuable applications. However, BSG protein fraction can be a valuable substrate for enzymatic hydrolysis to produce hydrolysates with biological properties and BSY contains numerous vacuole proteases, which can be used to obtain these hydrolysates. Thus, the objective of this work was to explore the valorization of these two brewing by-products, based on the production of BSG protein hydrolysates that present antioxidant properties. Response surface methodology was employed to optimize the hydrolysis of BSG proteins using BSY proteases, based on degree of hydrolysis (DH %), total phenolic content (TPC) and antioxidant activity (FRAP assay). Reversed-phase chromatography was also used to monitor the hydrolysis rate (HR %), and the potential presence of endogenous and other inhibitors of BSY proteases was also investigated. BSG protein hydrolysate prepared at 50 °C, pH 6, for 6 h and using an enzyme/substrate ratio of 0.29:1 U/mg presented maximum bioactivity with DH % of 17.10 %, TPC of 1.65 mg GAE/mL and FRAP value of 1.88 mg Trolox equivalent/mL. A good correlation was obtained between HR (%) and DH (%) (R 2 = 0.928). The experimental values agreed with the predicted values (p < 0.05), suggesting a good fit between the models and the experimental data. BSY proteases involved in the hydrolysis of BSG proteins are serine peptidases and metallopeptidases.



Similar content being viewed by others
References
Unicer (2013) Management report 60-1
Mussatto S, Dragone G, Roberto I (2006) Brewers’ spent grain: generation, characteristics and potential applications. J Cereal Sci 43(1):1–14
Celus I, Brijs K, Delcour JA (2007) Enzymatic hydrolysis of brewers’ spent grain proteins and technofunctional properties of the resulting hydrolysates. J Agric Food Chem 55(21):8703–8710
McCarthy AL, O’Callaghan YC, Connolly A, Piggott CO, FitzGerald RJ, O’Brien NM (2012) Phenolic extracts of brewers’ spent grain (BSG) as functional ingredients: assessment of their DNA protective effect against oxidant-induced DNA single strand breaks in U937 cells. Food Chem 134(2):641–646
Connolly A, Piggott CO, FitzGerald RJ (2013) Characterisation of protein-rich isolates and antioxidative phenolic extracts from pale and black brewers’ spent grain. Int J Food Sci Technol 48(8):1670–1681
Meneses NG, Martins S, Teixeira JA, Mussatto SI (2013) Influence of extraction solvents on the recovery of antioxidant phenolic compounds from brewer’s spent grains. Sep Purif Technol 108:152–158
Moreira MM, Morais S, Carvalho DO, Barros AA, Delerue-Matos C, Guido LF (2013) Brewer’s spent grain from different types of malt: evaluation of the antioxidant activity and identification of the major phenolic compounds. Food Res Int 54(1):382–388
Celus I, Brijs K, Delcour JA (2009) Fractionation and characterization of brewers’ spent grain protein hydrolysates. J Agric Food Chem 57(12):5563–5570
McCarthy AL, O’Callaghan YC, Connolly A, Piggott CO, FitzGerald RJ, O’Brien NM (2013) In vitro antioxidant and anti-inflammatory effects of brewers’ spent grain protein rich isolate and its associated hydrolysates. Food Res Int 50(1):205–212
Connolly A, Piggott CO, FitzGerald RJ (2014) In vitro α-glucosidase, angiotensin converting enzyme and dipeptidyl peptidase-IV inhibitory properties of brewers’ spent grain protein hydrolysates. Food Res Int 56:100–107
Yeast Handling/Processing (2014) United States Department of Agriculture
Hecht KA, O’Donnell AF, Brodsky JL (2014) The proteolytic landscape of the yeast vacuole. Cell Logist 4(1):e28023
Kuwabara Y, Nagai S, Yoshimitsu N, Nakagawa I, Watanabe Y, Tamai Y (1995) Antihypertensive effect of the milk fermented by culturing with various lactic acid bacteria and a yeast. J Ferment Bioeng 80(3):294–295
Roy MK, Watanabe Y, Tamai Y (2000) Yeast protease B-digested skimmed milk inhibits angiotensin-I-converting-enzyme activity. Biotechnol Appl Biochem 31(Pt 2):95–100
Chaves-Lopez C, Tofalo R, Serio A, Paparella A, Sacchetti G, Suzzi G (2012) Yeasts from Colombian Kumis as source of peptides with Angiotensin I converting enzyme (ACE) inhibitory activity in milk. Int J Food Microbiol 159(1):39–46
Vieira E, Brandao T, Ferreira IM (2013) Evaluation of Brewer’s spent yeast to produce flavor enhancer nucleotides: influence of serial repitching. J Agric Food Chem 61(37):8724–8729
Cupp-Enyard C (2008) Sigma’s non-specific protease activity assay-casein as a substrate. J Vis Exp 19(899). doi:10.3791/899
Vieira E, Rocha MAM, Coelho E, Pinho O, Saraiva JA, Ferreira IMPLVO et al (2014) Valuation of brewer’s spent grain using a fully recyclable integrated process for extraction of proteins and arabinoxylans. Ind Crops Prod 52:136–143
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72(1–2):248–254
Hsu K-C (2010) Purification of antioxidative peptides prepared from enzymatic hydrolysates of tuna dark muscle by-product. Food Chem 122(1):42–48
Ferreira IMPLVO, Eça R, Pinho O, Tavares P, Pereira A, Cecília Roque A (2007) Development and validation of an HPLC/UV method for quantification of bioactive peptides in fermented milks. J Liq Chromatogr Relat Technol 30(14):2139–2147
Herald TJ, Gadgil P, Tilley M (2012) High-throughput micro plate assays for screening flavonoid content and DPPH-scavenging activity in sorghum bran and flour. J Sci Food Agric 92(11):2326–2331
Jansen E, Ruskovska T (2013) Comparative analysis of serum (anti)oxidative status parameters in healthy persons. Int J Mol Sci 14(3):6106–6115
Bolumar T, Sanz Y, Aristoy MC, Toldrá F (2005) Protease B from Debaryomyces hansenii: purification and biochemical properties. Int J Food Microbiol 98(2):167–177
García-Carreño FL (1992) Protease inhibition in theory and practice. Biotechnol Educ 3(4):145–150
Fang Y-Z, Yang S, Wu G (2002) Free radicals, antioxidants, and nutrition. Nutrition 18(10):872–879
Hayashi R, Oka Y, Doi E, Hata T (1968) Activation of intracellular proteinases of yeast: Part I. Occurrences of inactive precursors of proteinases B and C and their activation part II. Activation and some properties of pro-proteinase C. Agric Biol Chem 32(3):359–366
Acknowledgments
This work received financial support from project UID/QUI/50006/2013-POCI/01/0145/FEDER/007265 with financial support from FCT/MEC through national funds and co-financed by FEDER, under the Partnership Agreement PT2020. One of the authors (E. F. Vieira) wishes to thank the FCT Grant SFRH/BD/81845/2011. To all financing sources, the authors are greatly indebted.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Compliance with ethics requirements
This article does not contain any studies with human or animal subjects.
Additional information
Elsa Vieira and Juliana Teixeira have contributed equally to this experimental work.
Rights and permissions
About this article
Cite this article
Vieira, E., Teixeira, J. & Ferreira, I.M.P.L.V.O. Valorization of brewers’ spent grain and spent yeast through protein hydrolysates with antioxidant properties. Eur Food Res Technol 242, 1975–1984 (2016). https://doi.org/10.1007/s00217-016-2696-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-016-2696-y