Abstract
A novel UV apparatus based on Dean vortex technology is designed for inactivating bacteria in milk. In this apparatus, the milk flows through a helical quartz tube coiling around an electrodeless UV lamp (EUL) with a radio frequency of 2.65 MHz. Flow rate, inner diameter of quartz tube, different UV sources, and different types of bacteria have been found as the key factors for the valuable effects on bacterial inactivation. The EUL apparatus worked more efficiently in the UV inactivation of the predetermined populations of milk-related bacteria than the conventional low-pressure high-intensity mercury lamp. When the UV dose of 21.3 mJ/cm2 was applied, the numbers of all the bacteria were reduced by more than 6 log10 with a flow rate of 28.8 L/h and a tube’s inner diameter of 1.5 mm. Dean vortices were formed in the milk flow during the UV processing and played an important role in the UV inactivation of the bacteria. Another inactivation test with the apparatus applying the UV dose of 21.3 mJ/cm2 was also done with raw cow’s milk containing indigenous microorganisms, including Salmonella and Shigella spp., Listeria monocytogenes, Staphylococcus spp., Enterobacteriaceae, lactic acid bacteria, pseudomonads, and the total aerobic bacteria were reduced by approximately 3–4 log10. In short, the EUL apparatus requires smaller energy, occupies less space, and has simpler operating procedures than thermal pasteurization. Thus, the novel method provides a viable alternative to thermal pasteurization of milk for improving the microbial safety of milk and extending its shelf life.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Non-thermal processing technologies applied to food preservation have attracted increasing research interest in the past two decades as they provide a valid alternative to conventional thermal processing and produce safe but minimally processed food with a minimum of product changes (taste, ingredients, etc.) [1]. In the case of milk and dairy products, the antimicrobial effect of novel technologies, such as high-pressure treatment [2–4] and pulsed electric fields [5, 6], have been demonstrated. Another approach with considerable antimicrobial potential involves the use of UV radiation. It can be applied for disinfection of water, surfaces of fresh fruits or lettuce surfaces [7, 8], and liquids such as fruit juices, apple cider, sugar syrup, or milk [9–13].
Although UV-C light (200–280 nm) can inactivate all bacteria in milk, the efficiency of the process is rather low using traditional UV disinfection apparatus. The reason is that UV-C light only penetrates a much shorter depth below the surface of milk than pure water [14]. Due to the high percentage of suspended solids, i.e., protein and fat, in milk, the depth of penetration in milk is relatively small (absorption of 99% within one millimeter). Therefore, to minimize absorption, a special conduction of the liquid flow is being used in most applications. Some applications working with thin films [10] or capillaries [15, 16] were demonstrated to be effective for the inactivation of inoculated microorganisms into milk substrates. The microorganisms studied in milk include Listeria monocytogenes [10], Staphylococcus aureus [16], Mycobacteium avium subsp. Paratuberculosis [15], and others.
A novel apparatus is designed with a helical quartz capillary coiling around a high-intensity electrodeless UV lamp. The capillary can help the liquid flow to form secondary vortices, known as “Dean vortices”, and thus enhance the radial mixture of the fluid in a laminar flow field [17]. Therefore, even in milk with low UV penetration depths, all fluid elements can be treated. The previous studies published with regard to UV treatment of milk focused on only several individual bacterial species inoculated in milk. The main objective of this study was to investigate the inactivation of more kinds of most often found pathogenic and spoilage microorganisms inoculated in ultra-high temperature (UHT) milk and even the inactivation of the indigenous microorganisms in raw cow’s milk using this novel UV apparatus. The typical pathogenic microorganisms inoculated in milk were different strains of the following bacteria: Listeria monocytogenes, Escherichia coli, Staphylococcus aureus, Salmomella enteria, Shigella flexneri, and Mycobacterium tuberculosis, while the typical spoilage microorganisms inoculated in milk were different strains of Pseudomonas aeruginosa and Lactococcus lactis.
Materials and methods
UV apparatus
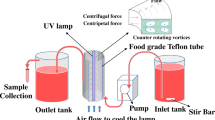
As shown in Fig. 1, the essential part of the lab-scale UV apparatus is a helical quartz tube coiling around an 80 W low-pressure electrodeless UV lamp. The electrodeless UV lamp is started by delivering radio frequency (RF) power of 2.65 MHz from the RF generator (Fig. 2a) into the induction coil (D) in the lamp. The electric current passing through the induction coil generates a magnetic field. Then, the mercury–argon gas (C) inside the quartz bulb (B) is excited and ionized in the magnetic field. Therefore, the gas discharge occurs and a large amount of UV light at 253.7 nm is emitted, which is similar to conventional low-pressure mercury lamps. A cooling fin (E) and an air chiller (F) are used to cool the UV lamp [18].
As shown in Fig. 1, the milk is pumped from a container using a small peristaltic pump (6–600 rpm, Cole Parmer Inc.). The milk passes through the helical quartz tube twisting around the UV lamp in a spiral motion with a laminar flow rate. The flow rate of the milk is controlled with a speed controller made by the same pump company.
Milk products and preparation
Full cream UHT cow’s milk (Mengniu Dairy Ltd., Neimenggu, China) was purchased from a local supper market and was used as a sterile medium for microbiological investigations. Full cream raw cow’s milk was obtained from Guangming dairy farm at Shenzhen, China, as the source of indigenous milk microorganisms.
Bacterial preparation and counting methods
Listeria monocytogenes (ATCC 7644, ATCC 19114, ATCC 19111, ATCC 15313), Mycobacterium tuberculosis (ATCC 25177, ATCC 27294), Escherichia coli (ATCC 25922, ATCC 15597, ATCC 11229), Staphylococcus aureus (ATCC 25923, ATCC 6538, ATCC 49444), Salmonella Typhimurium (ATCC 14028, ATCC 6539, ATCC 19585), Shigellaflexneri (ATCC 12022, ATCC 29903, ATCC 9199), Pseudomonas aeruginosa (ATCC 27853, ATCC 10145, ATCC 9027), and Lactococcus lactis (ATCC 19435, ATCC 7962, ATCC 29146) were obtained from the American Type Culture Collection, Manassas, VA, USA. All the strains were isolated from different representative sources. The strains were inoculated into tryptone soya broth (pH 7.0 ± 0.2) supplemented with 0.6% yeast extract (TSYEB) at 30 °C under aerobic conditions and shaking at 200 rpm for 24 h except the L.lactis strains were grown in de Man, Rogosa and Sharpe (MRS) broth under anaerobic and stationary conditions for 24 h. The M. tuberculosis strains were grown at 35 °C for 14 days in Middlebrook and Cohn 7H-9 liquid medium (Difco Micro. Inc.) containing 0.05% Tween 80. All cultures were subcultured twice, and then, the cells were taken in stationary growth phase. Then, the cells were centrifuged at 7,500 g for 10 min, washed three times with a sterilized phosphate buffer solution (pH 7.6) and finally added to the UHT milk to achieve the required final concentration (106–107 CFU/mL). For enumeration of the bacteria before and after UV treatment, the milk samples were immediately plated out on tryptone soya agar (pH 7.0 ± 0.2) supplemented with 0.6% yeast extract (TSYEA) and incubated at 37 °C for 24 h, except the milk samples containing L.lactis were immediately plated out on MRS agar and incubated at 37 °C for 48 h, and the milk samples containing M. tuberculosis were immediately plated out on Middlebrook 7H-10 agar and incubated at 35 °C for the appropriate length of time [19, 20].
The indigenous microorganisms [up to 104 colony forming units (CFU) per mL] in two samples of raw milk were, respectively, grown by incubation at 37 °C for 24 h and at 4 °C for 24 h and then were mixed for producing a diversified microbial population of up to 109 CFU per mL. Subsequently, this milk was filtered through a 50-μm mesh and used as inoculum. Subsamples of this milk were applied to inoculate UHT milk in a 1:50 ratio. The UHT milk inoculated with incubated raw milk was used for the inactivation experiments.
Comprehensive microbiological analyses of the raw milk samples were accomplished using spread plate method with the following agar types: Total bacterial counts on plate count agar (PCA) as described by Mol [21]; Lactic acid bacteria on De Man, Rogosa, and Sharpe agar (CM0361, Oxoid Ltd, Basingstoke, Hampshire, UK) [22]; Pseudomonads on Ps. selective agar (CM0559 and SR0103, Oxoid Ltd) [23, 24]; Salmonella and Shigella counts on Salmonella–Shigella (SS) agar (Difco) [25]; Staphylococcus counts on Baird Parker agar (CM0275 and SR54, Oxoid Ltd) [26]; L. monocytogenes on Listeria selective agar (LSA, CM0856 and SR0140, Oxoid Ltd.) [10]; Enterobacteriaceae on Violet Red Bile Dextrose agar (VRBD) (Huankai, Guangdong, China) [27].
UV inactivation experiments
The electrodeless UV lamp is kept on for 10 min for preheating before the milk processing, while the air chiller is started. Flow rate and inner diameter of the quartz tube were optimized in the UV processing to achieve the maximum microbial reduction. The effect of milk flow rate on UV inactivation of bacteria was examined by cycling the milk for 1, 2, 3, 4 times at a flow rate of 6.8 L/h, and for 3, 6, 9, 12 times at 17.3 L/h, and for 5, 10, 15, 20 times at 28.8 L/h. Also, tube’s inner diameters of 1.5, 2.0, and 3.0 mm were examined. Their respective tube cross-sectional areas were 1.8, 3.1, and 7.1 mm2, and their related volumes were 6.8, 8.9, and 13.3 mL. The Dean number was calculated using the following equation: \( {\text{De}} = {{\rho vD} \mathord{\left/ {\vphantom {{\rho vD} {\mu \cdot \sqrt {D/2R} }}} \right. \kern-\nulldelimiterspace} {\mu \cdot \sqrt {D/2R} }}, \) where ρ is the density of the milk fluid (kg/m3), v is the average velocity of milk flow (m/s), D is the diameter of the tubing (m), μ is the viscosity of the fluid (N s/m2), and R is the radius of curvature of the path of the helical tube (m) [28]. The values used to calculate Dean numbers for each operating mode were as follows: ρ = 1,029 kg/m3; v is shown in Table 1; D = 0.0015, 0.002 or 0.003 m for different tube diameters; μ = 0.002725 Ns/m2; R = 0.025 m. The calculated Dean numbers are also shown in Table 1. In the optimization experiments, the milk was inoculated with pure cultures of E. coli (a mixture of three strains) and well mixed by a stir plate (Model 310T, Fisher Scientific). A better microbial reduction in milk would be obtained by multiple passes through the apparatus; therefore, the apparatus needed to be cleaned and sanitized after each pass. A volume 200 ppm hypochlorite solution was used and then flushed with sterilized water to remove the chlorine residue [11]. Cleaning time was less than 2 min, and sterility check for the apparatus was made after each cleaning process. One liter of inoculated milk was pumped from one sterilized container and passed through the UV apparatus to another sterilized container and then repeated the process until the predetermined times were completed. After certain times, 50 mL of milk was collected from the UV apparatus and immediately assessed for microbial load. The experiments were carried out in an aseptic condition of a laminar flow class II biological safety cabinet (Model: LB2-3B1, Esco Technologies Inc., Singapore). As controls, identical samples were pumped through the UV reactors and held for the same time as required for irradiation without UV light. The control sample was enumerated both before and after each experiment in order to confirm that significant die-off did not occur over the period of each experiment. The measurement of incident UV intensity was made at a 254 nm wavelength, using a radio meter with a UVX-25 sensor (UVP Inc., CA, USA). The average intensity within the milk sample was calculated by an integration of Lambert–Beer law over the sample thickness. The UV dose was calculated using the equation UV dose (mJ/cm2) = UV intensity × exposure time. The UV intensity was calculated by the method described by Quintero-Ramos et al. [11]. It was determined by multiplying the sensor readings by the radial factor, reflection factor, and the absorption factor, which for this milk was 0.3046. The exposure time was equal to the volume of the helical quartz tube divided by the milk flow rate and multiplied by the number of cycling times. The results were shown in Table 2. To avoid air oxidation of the milk, the experiments were carried out in a nitrogen gas environment at room temperature (25 °C). After each pass through the UV apparatus, milk temperature would be raised by 2–3 °C. Therefore, a high-efficiency heat exchanger and an online thermocouple were used to keep the milk temperature at 25 ± 2 °C after each UV treatment. In this way, the milk heated by the UV treatment energy could not reach a temperature sufficient for heat inactivation of microorganisms. Survivors were enumerated both before and after each UV treatment by plate counting. Reduction in bacteria was expressed as log (N 0 /N), where N 0 and N were the concentrations of bacteria before and after irradiation, respectively. All experiments were done three times in duplicate. The results were expressed as the mean, and the standard errors were calculated.
For comparing the performance of the self-made electrodeless UV lamp (EUL) with that of the conventional low-pressure high-intensity mercury lamp (LHML), UV inactivation of different bacteria was also investigated. The EUL had a typically operating life span of 20,000–30,000 h and an incident UV intensity of 101.1 mW/cm2, while the conventional LHML (UVMax, Model C, Trojan Technologies Inc.) had only about 5,000–10,000 h and about 51.6 mW/cm2, respectively. The milk was inoculated with the pure cultures of multiple strain mixtures from eight varieties of bacteria to inoculum concentration of about 106–107 CFU/mL, respectively, and then processed in the tube’s inner diameter of 1.5 mm with the flow rate of 28.8 L/h for 10, 15, 20 times. All experiments were done at least three times in duplicate. Survivors were enumerated both before and after each UV treatment by plate counting as described above. The results were expressed as log10 (CFU per mL), where counts were the means of the three replicate trials. Analysis of variance was applied to compare the effects of the different UV sources, the UV doses, and the types of microorganisms treated, with Tukey’s Studentized Range (honestly significant difference) test at significant levels of α = 0.05 in Minitab software (Minitab Inc., States College, Pa.). The purpose was to determine the statistical significant differences between samples means.
After each experiment, the apparatus was immediately washed with 25 °C water for 20 min, cleaned with 60 °C NaOH solution (1%) for 15 min, and then with 60 °C heated water for 20 min. Finally, the helical tube was sterilized by autoclave at 121 °C for 20 min.
Energy consumption
The actual amount of energy needed for the treatment with the novel UV apparatus was the result of the following calculation. The energy for it based on using 80 W high-pressure UV lamp with a milk flow rate of 28.8 L/h (0.0228 m3/h), cycling for 20 times is E uv = 20 × 80 × 10−3 kW/(0.0288 m3/h) = 55.6 kW h/m3 or 194.2 kJ/kg.
Results and discussion
Effect of flow rate on microbial reduction
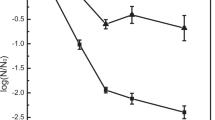
Figure 3 indicated that the reduction in E. coli was related to the flow rate of the milk through the UV apparatus with the tube of 1.5 mm in inner diameter. As the flow rate was increased, the E. coli reduction went up at the same UV dose. When operating at the flow rate of 6.8 L/h with the UV dose of 21.3 mJ/cm2, approximately 3.9 log10 reduction in E. coli was achieved. At the flow rate of 17.3 L/h, the E. coli reduction increased to approximately 6 log10 for the same UV dose of 21.3 mJ/cm2. At the flow rate of 28.8 L/h, the E. coli reduction further increased to approximately 6.5 log10 reduction at the same UV dose. Therefore, the following experiments were conducted at the flow rate of 28.8 L/h.
The effect of UV irradiation on microorganisms may depend on the UV dose, UV intensity, the absorptive properties and flow patterns of the mediums in which the organism are suspended, and the species of microorganisms [29]. Selecting proper flow patterns in milk flow is important for UV inactivation of microorganisms in milk. Matak et al. [10] reported that a proper turbulent flow in the Cidersure 3,500 apparatus could lead to the increase in the bacterial reduction rate in milk at the same UV dose, and the Reynolds number related with the turbulent flow was 5,187. In our study, the calculated Reynolds numbers for 6.8, 17.3, and 28.8 L/h were 595, 1,513, and 2,517, which could be translated into laminar and transient flow. According to our calculated Reynolds number, there might be no turbulent flow in the helical quartz tube, but the E. coli reduction rates significantly increased as the flow rate increased from 6.8 to 28.8 L/h. As we know, the helical quartz tube has helped the liquid flow to form the Dean vortices and thus has enhanced the radial mixture of the fluid in a laminar flow field [17]. Similar to the function of a critical Reynolds number for judging whether a fluid flow in a straight pipe is laminar or turbulent, a critical Dean number is utilized to determine whether the fluid flow in the curved pipe forms Dean vortices. It is proposed by several authors that the critical Dean number for non-Newtonian fluid is 150 [17, 30, 31]. In our experiments, the calculated Dean numbers for the flow rates of 6.8, 17.3, and 28.8 L/h with the 1.5-mm tube were 103, 262, and 436, respectively (Table 2). As the result, the E. coli reduction could only achieve approximately 4 log10 units at 6.8 L/h. However, for 17.3 and 28.8 L/h, the calculated Dean numbers were much greater than the critical one and more than 6 log10 reduction in E. coli could be achieved. It could be concluded that the strong mixing of the liquid caused by Dean vortices played an important role in the UV inactivation of the bacteria.
Effect of inner diameter of quartz tube on microbial reduction
Figure 4 demonstrated that the inner diameter of quartz tube had a great effect on UV inactivation of E. coli with the milk flow rate of 28.8 L/h. As the quartz tube inner diameter decreased, the E. coli reduction increased at the same UV dose. When 3.0-mm tube was applied, the achieved E. coli reduction was approximately 4.0 log10 with the highest UV dose of 20.8 ± 0.5 mJ/cm2. At 2.0 mm, the E. coli reduction dramatically increased to approximately 6.2 log10 units at the UV dose of 20.8 ± 0.5 mJ/cm2. Yet, only a slight increase appeared in the E. coli reduction with the 1.5-mm tube at the UV dose of 20.8 ± 0.5 mJ/cm2 compared with the 2.0-mm tube.
Comparison of the two factors—tube’s inner diameter and flow rate
At 1.5-mm tube with 17.3 L/h and 3.0-mm tube with 28.8 L/h, the calculated Dean numbers were 262 and 313 and their E. coli reductions were 6.0 log10 and 3.9 log10 units, respectively. Clearly, the results implicated that the UV inactivation of E. coli was much lower in 3.0-mm tube experiment although stronger mixing it had, compared with 1.5-mm tube test. So, the conclusion could be that increasing the inner diameter of the tubes led to the decreasing of the E. coli reduction. According to Lambert–Beer law, the absorption of the UV irradiation in its passage through a liquid increased with increasing depth of the liquid, which resulted in a decrease in UV irradiation on bacteria and thus led to the decreased UV inactivation of bacteria. Therefore, the 1.5-mm tube was chosen for the systems studied for UV processing of milk.
Inactivation of bacteria inoculated in UHT milk with different UV sources
Table 3 summarized UV inactivation of all the test strains inoculated in UHT milk with the electrodeless UV lamp (EUL) and the low-pressure high-intensity mercury lamp (LHML) at different UV doses. The viable count reduction results were for multiple strain mixtures from eight varieties of bacteria. The 1.5-mm tube was used in this UV processing, and the milk was circulated in the tube at the flow rate of 28.8 L/h for 10, 15, and 20 times, respectively. The results indicated that the inactivation of each bacterium increased as the UV dose rose up from 10.7 to 21.3 mJ/cm2 (P < 0.05) and, UV inactivation with the EUL was more effective than that with the LHML. At the UV dose of 10.7 mJ/cm2, the EUL had an average increase of 0.9 log10 compared with the LHML, when 16.0 mJ/cm2, it was 1.4 log10 and when 21.3 mJ/cm2, 2.4 log10, the highest increase was achieved.
When the EUL was used as the UV source and the UV dose increased from 10.7 to 16.0 mJ/cm2, approximately 2–4 log10 reduction in L. lactis, L. monocytogenes, and M. tuberculosis, 3–5 log10 reduction in S. aureus, S. typhimurium, E. coli, and P. aeruginosa, and 3.5–6.5 log10 reduction in S. flexneri were achieved. Furthermore, more than 6 log10 reduction in all test bacteria could be achieved with a UV dose of 21.3 mJ/cm2. It was also found that the test bacteria’s resistance varied with the bacterial species. The resistance levels to the UV irradiation were listed in a decreasing order for the following test bacteria: L. lactis, L. monocytogenes, M. tuberculosis, S. aureus, S. typhimurium, E. coli, P. aeruginosa, S. flexneri (Note: the levels of resistance among L. lactis, L. monocytogenes, and M. tuberculosis and those among S. aureus, S. typhimurium, and E. coli did not significantly differ at the level of P < 0.05).
The EUL was more efficient in the UV inactivation of various test bacteria compared with the LHML. Although both UV lamps irradiated UV light mainly at 253.7 nm, the EUL had an incident intensity of 101.1 mW/cm2, about two times more than that of the LHML. Moreover, the higher the UV dose was applied, the larger differences in UV inactivation the both lamps had. These results were in good agreement with the research of Sommer, Haider, Cabaj, Pribil, and Lhotsky [32]. According to their reports, inactivation of the E. coli strains with a high UV intensity for a short amount of time was higher than that with a lower UV intensity for a longer amount of time at the same UV dose. Furthermore, the differences of E. coli inactivation between the high and low UV intensities were greater at UV doses of 8–10 mJ/cm2 than those at lower UV doses of 4–6 mJ/cm2. This effect might be due to the repair enzymes of the cells, which were significantly more influenced by high intensities.
Our study also demonstrated that UV resistance of microorganisms varied from species to species (Table 3). These results were in agreement with the work of Jay [33] and Rowan et al. [20]. Jay reported that gram-positive bacteria were more resistant to the effects of UV light than gram-negative bacteria. According to Rowan et al., L. monocytogenes was the most resistant to UV inactivation. Our data are also in agreement with the results achieved by Chang et al. [34], i.e., S. aureus, S. typhimurium, E. coli, and S. sonnei showed the same resistance to UV light at the level P < 0.05. In our tests, the gram-positive bacteria, L. lactis and L. monocytogenes, and M. tuberculosis were the most resistant to the UV irradiation among the test bacteria. However, more than 3 log10 reduction in L. lactis and 4 log10 reduction in L. monocytogenes and M. tuberculosis were achieved for UV dose of 16.0 mJ/cm2 with the milk flow rate of 22.8 L/h in the helical quartz tube with the EUL. The three bacteria could be reduced by more than 6 log10 reduction when the UV dose increased to 21.3 mJ/cm2. The reason was that the apparatus had promoted the forming of Dean vortices in the milk flow, which had brought the bacteria to the milk surface for enough exposure to high-intensity UV light irradiated by the EUL. Therefore, the UV apparatus has created the possible killing of the bacteria even in the opaque milk.
Inactivation of indigenous microorganisms in raw milk
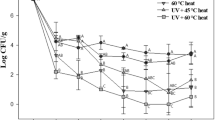
Figure 5 showed the UV inactivation of indigenous microorganisms in raw milk with different cycling times of 5, 10, 15, and 20 in the 1.5-mm tube at 28.8 L/h. The initial microbial counts enumerated before UV treatment suggested that the initial contamination was rather high at approximately 1.2 × 104 CFU/mL total aerobic bacteria, 2.3 × 103 CFU/mL Staphylococcus spp., 6.1 × 103 CFU/mL Enterobacteriaceae, 1.0 × 103 CFU/mL Salmonella and Shigella spp., 1.1 × 103 CFU/mL lactic acid bacteria, 8.0 × 102 CFU/mL Listeria monocytogenes, and 6.0 × 102 CFU/mL pseudomonads. No matter, the UV dose was 10.7 or 16.0 mJ/cm2, approximately 2–3 log10 reduction in each microbial count was achieved. No survivors were enumerated in the milk samples at the highest UV dose of 21.3 mJ/cm2. It showed that the lactic acid bacteria were the most resistant to the UV processing at different UV doses as it was depicted in Table 3, but other selective counts of the indigenous microorganisms did not differ at the level of P < 0.05.
UV inactivation of indigenous microorganisms in raw milk. PCA total aerobic plate counts on plate count agar, MRS the lactic acid bacterial count on de Man, Rogosa, and Sharp agar, VRBD Enterobacteriaceae count on Violet Red Bile Dextrose agar, SS Salmonella and Shigella counts on Salmonella–Shigella agar, BP Staphylococcus count on Baird Parker agar, LSA the Listeria monocytogenes count on Listeria selective agar, PSA the pseudomonad count on Pseudomonas selective agar
The UV apparatus was quite efficient in inactivating the pure cultures of pathogenic and spoilage microorganisms inoculated in UHT milk, but the inactivation of the indigenous microorganisms in raw milk was not so efficient. For the UV dose of 10.7 mJ/cm2, only about 2 log10 reduction in the indigenous microorganisms in the raw milk occurred, while more than 3 log10 reduction in each bacterium inoculated in UHT milk was achieved except Lactobacillus lactis, of which the reduction was 2.1 log10 units. When the UV dose increased to 21.3 mJ/cm2, the reductions in the indigenous microorganisms were still less than those of the inoculated bacteria. The reasons for the above differences can only be speculated on. It may be possible that a subpopulation of microorganisms have their UV resistance genes turned on and thus may accelerate the production of resistance proteins such as RecA and other proteins involved in dark repair. Additionally, the ecology of the indigenous microorganisms in raw milk is probably very diverse. Selective counts of the indigenous microorganisms were isolated and enumerated species from the same genus, but each of the inoculated bacteria was cultivated from only several strains of the same species. Therefore, bacterial species of the indigenous microorganisms with higher UV resistances than the pure cultures used in the inactivation experiments might have been presented, which lead to an increased survival after UV irradiation.
However, obvious reductions from about 1 × 103 CFU/mL of Salmonella and Shigella spp. or Listeria monocytogenes to below detectable limits occurred in the UV processing of the indigenous microorganisms for UV doses above 16.0 mJ/cm2. Similarly, the counts of Staphylococcus spp., Enterobacteriaceae, lactic acid bacteria, or pseudomonads were reduced from 1 × 103–1 × 104 CFU/mL to below 10 CFU/mL or below detectable limits for UV doses above 16.0 mJ/cm2. These were quite notable reductions and may thus help for the microbial safety and increased shelf life of milk.
The US Food and Drug Administration (USFDA) requires milk to be pasteurized in order to be safe for drink. One standard for the high temperature, short time (HTST) pasteurization was designed to achieve a 5 log reduction, killing 99.999% of the number of viable microorganisms in milk, recommended by the USFDA. When the UV dose of 21.3 mJ/cm2 was applied, even the most UV-resistant pathogen, L. monocytogenes was reduced by more than 6 log10 and higher reductions in the other test bacteria were also achieved. This result was higher than the 5 log10 reduction required by the USFDA. Applying higher UV dose could lead to higher reduction of pathogens in milk, and thus the UV apparatus could further satisfy higher requirements for pathogens in milk.
In comparison, the energy requirement for the HTST processing is between 217 and 228 kJ/kg milk (72–75 °C) and that for the UHT processing ranges between 573 and 667 kJ/kg milk [35]. Furthermore, the latter two processes (HTST and UHT) have to have three stages including heating, sterilization, and cooling, while the UV irradiation processing needs only one.
Dean vortices were demonstrated to be formed in the milk flow during the UV processing, which had a great effect on the UV inactivation of the bacteria. It has also been found out that the EUL with higher UV intensity was more efficient in the UV inactivation of the test bacteria than the conventional LHML. Even though L. lactis, L. monocytogenes, and M. tuberculosis among the test bacteria were the most resistant to the UV processing, more than 6 log10 reduction in all the test bacteria could still be achieved when the UV dose was increased to 21.3 mJ/cm2 with the milk flow rate of 28.8 L/h going through the 1.5-mm tube.
Conclusions
This novel apparatus could achieve a greater than 6 log10 reduction in the pure cultures of all the milk-related bacteria and 3–4 log10 reduction of the indigenous bacteria in the raw cow’s milk. Dean vortices were formed in the milk flow during the UV processing and played an important role in the UV inactivation of the bacteria. The apparatus has potential to improve the safety and extend the shelf life of milk, for it also needs less energy than for the thermal pasteurization and requires less space and has simpler processing procedures. It could be used for the reduction of milk-related microorganisms as a viable alternative method to thermal pasteurization. Further research should assess lipid and protein oxidation and organoleptic properties of the irradiated milk. UV inactivation of various raw milks from different sources and the effect of milk fat content on UV inactivation efficiency should also be further studied.
References
Elmnasser N, Dalgalarrondo M, Orange N, Bakhrouf A, Haertle T, Federighi M, Chobert JM (2008) Effect of pulsed-light treatment on milk proteins and lipids. J Agric Food Chem 56:1984–1991
De Ancos B, Cano MP, Gómez R (2000) Characteristics of stirred low-fat yoghurt as affected by high pressure. Int Diary J 10:105–111
Guan D, Chen H, Hoover DG (2005) Inactivation of Salmonella typhimurium DT 104 in UHT whole milk by high hydrostatic pressure. Int J Food Microbiol 104:145–153
López-Pedemonte T, Roig-Sagueś AX, De Lamo S, Gervilla R, Guamis B (2007) High hydrostatic pressure treatment applied to model cheeses made from cow’s milk inoculated with Staphylococcus aureus. Food Control 18:441–447
Angersbach A, Heinz V, Knorr D (2000) Effects of pulsed electric fields on cell membranes in real food systems. Innovative Food Sci Emerg Technol 1:135–149
Walkling-Ribeiro M, Noci F, Cronin DA, Lyng JG, Morgan DJ (2009) Antimicrobial effect and shelf-life extension by combined thermal and pulsed electric field treatment of milk. J Appl Microbiol 106:241–248
Fino VR, Kniel KE (2008) UV light inactivation of hepatitis A virus, Aichi virus, and feline calicivirus on strawberries, green onions, and lettuce. J Food Prot 71:908–913
Lagunas-Solar MC, Pina C, MacDonald JD, Bolkan L (2006) Development of pulsed UV light processes for surface fungal disinfection of fresh fruits. J Food Prot 69:376–384
Geveke DJ (2005) UV inactivation of bacteria in apple cider. J Food Prot 68:1739–1742
Matak KE, Churey JJ, Worobo RW, Sumner SS, Hovingh E, Hackney CR, Pierson MD (2005) Efficacy of UV light for the reduction of Listeria monocytogenes in goat’s milk. J Food Prot 68:2212–2216
Quintero-Ramos A, Churey JJ, Hartman P, Barnard J, Worobo RW (2004) Modeling of Escherichia coli inactivation by UV irradiation at different pH values in apple cider. J Food Prot 67:1153–1156
Stother B (1999) UV disinfection in liquid sugar. Int Sugar J 101:361–363
Tran MTT, Farid M (2004) Ultraviolet treatment of orange juice. Innovative Food Sci Emerg Technol 5:495–502
Shama G (1999) Ultraviolet light, p. 2208–2214. In: Robinson RK, Batt C, Patel P (eds) Encyclopedia of food microbiology-3. Academic Press, London
Altic LC, Rowe MT, Grant IR (2007) UV light inactivation of Mycobacterium avium subsp. paratuberculosis in milk as assessed by FastplaqueTB phage assay and culture. Appl Environ Microbiol 73:3728–3733
Krishnamurthy K, Demirci A, Irudayaraj JM (2007) Inactivation of Staphylococcus aureus in milk using flow-through pulsed UV-light treatment system. J Food Sci 72:233–239
Hille P, Vehrenkamp R, Schulz-Dubois EO (1985) The development and structure of primary and secondary flow in a curved square duct. J Fluid Mech 151:219–241
Proud JM, Johnson SG (1981) Electrodeless ultraviolet light source. US patent 4,427,921
David HL, Jones WD, Newman CM (1971) Ultraviolet light inactivation and photoreactivation in the mycobacteria. Infect Immun 4:318–319
Rowan NJ, MacGregor SJ, Anderson JG, Fouracre RA, Mcilvaney L, Farish O (1999) Pulsed-light inactivation of food-related microorganisms. Appl Environ Microbiol 65:1312–1315
Mol S (2005) Preparation and the shelf-life assessment of ready–to-eat fish soup. Eur Food Res Tech 220:305–308
Scolari G, Vescovo M, Zacconi C, Bonadé A (2004) Influence of Lactobacillus plantarum on Staphylococcus aureus growth in a fresh vegetable model system. Eur Food Res Tech 218:274–277
Massa S, Fanelli M, Brienza MT, Sinigaglia M (1997) The bacterial flora in bottled natural mineral water sold in Italy. J Food Qual 21:175–185
UK NHS/NPHSW/HPA (2007) Identification of glucose nonfermenting gram-negative rods, National Standard Method BSOP ID17i2. London, UK: National Health Service, Standards Unit, Evaluations and Standards Laboratory, Health Protection Agency, Centre for Infections, National Public Health Service for Wales
Krieg NR (1981) Enrichment and isolation. p. 112–142. In: Gerhardt P (ed) Manual of methods for general bacteriology. American Society for Microbiology, Washington, DC
Smiddy MA, Martin JE, Huppertz T, Kelly AL (2007) Microbial shelf-life of high-pressure-homogenised milk. Int Dairy J 17:29–32
Franz CMAP, Specht I, Cho GS, Graef V, Stahl MR (2009) UV-C-inactivation of microorganisms in naturally cloudy apple juice using novel inactivation equipment based on Dean vortex technology. Food Control 20:1103–1107
Berger SA, Talbot L, Yao LS (1983) Flow in curved pipes. Annu Rev Fluid Mech 15:461–512
Guerrero-Belán JA, Barbosa-Cánovas GV (2004) Review: advantages and limitations on processing foods by UV light. Food Sci Technol Int 10:137–147
Bara B, Nandakumar K, Masliyah JH (1992) An experimental and numerical study of the Dean problem: flow development towards two-dimensional multiple solutions. J Fluid Mech 244:339–376
Fellouah H, Castelain C, El Moctar AO, Peerhossaini H (2006) A numerical study of Dean instability in Non-Newtonian fluids. J Fluid Eng 128:34–41
Sommer R, Haider T, Cabaj A, Pribil W, Lhotsky M (1998) Time dose reciprocity in UV disinfection of water. Water Sci Technol 38:145–150
Jay JM (1997) Radiation protection of foods and nature of microbial radiation resistance, p. 373–394. In: Jay JM (ed) Modern food microbiology. Springer Science and Business Media, New York
Chang JCH, Ossoff SF, Lobe DC, Dorfman MH, Dumais CM, Qualls RG, Johnson JD (1985) UV inactivation of pathogenic and indicator microorganisms. Appl Environ Microbiol 49:1361–1365
Chandarana DI, Frey BC, Stewart LE, Mattick JF (1984) UHT milk processing-effect on process energy requirements. J Food Sci 49(3):977–978
Acknowledgments
The authors express thanks to the experts from China National Research Institute of Food Fermentation Industries for their continued support throughout the duration of this work and Pro. Dayue Fan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lu, G., Li, C. & Liu, P. UV inactivation of milk-related microorganisms with a novel electrodeless lamp apparatus. Eur Food Res Technol 233, 79–87 (2011). https://doi.org/10.1007/s00217-011-1498-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-011-1498-5