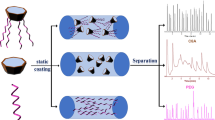
Abstract
This work presents the first example of the utilization of polar ester group functionalized pillar[6]arene (P6A-C10-OAc) as a stationary phase for capillary gas chromatographic (GC) separations. The statically coated P6A-C10-OAc column showed a high column efficiency of 5393 plates/m and moderate polar nature. Its resolving capability and retention behaviors were investigated for a mixture of 20 analytes and more than a dozen isomers from apolar to polar in nature. As evidenced, the P6A-C10-OAc column achieved high-resolution separations of all the analytes and good inertness. Importantly, it exhibited distinctly advantageous performance for high resolution of the challenging isomers of xylenes, diethylbenzenes, ethyltoluenes, and halobenzenes over the commercial HP-5 (5% phenyl dimethyl polysiloxane), HP-35 (25% phenyl dimethyl polysiloxane), and PEG-20M (polyethylene glycol) columns.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gas chromatography (GC) has been widely used in various fields including chemical, pharmaceutical, and petroleum industries because of its low cost, short analysis time, and high selectivity [1, 2]. The highly effective separation of analytes with a close nature in GC depends on a stationary phase with advantageous selectivity. Therefore, more and more stationary phases such as polymers [3], ionic liquids (ILs) [4,5,6], metal-organic frameworks (MOFs) [7, 8], macrocycles, covalent organic frameworks (COFs) [9, 10], and carbon nanomaterials [11, 12] are developed to be applied in GC. Xylene, halobenzene, and benzaldehyde are important industrial raw materials, which are widely used in the chemical industry. Besides, they are important environmental pollutants, and their efficient separation is a challenging task in the field of analysis. Due to their very similar physicochemical properties, it is difficult to effectively separate these compounds and their isomers with conventional stationary phases [13]. Therefore, it is necessary to develop new stationary phases for gas chromatography with high selectivity for these target analytes.
Pillararenes, as a new class of macrocyclic hosts after crown ethers [14,15,16] cyclodextrin [17,18,19], calixarene [20], and cucurbituril [21,22,23], are made up of 1,4-disubstituted hydroquinone units linked by methylene bridges at the 2,5-positions and have received much more attention since they were synthesized by Ogoshi and co-workers in 2008 [24]. The number of repeated hydroquinone subunits determines the size of the cavity and the exist of the benzene rings gives the pillararenes electron-rich cavity. For example, pillar[6]arenes (approximately 6.7 Å) have larger cavities than pillar[5]arenes (approximately 4.7 Å), and the difference makes pillar[6]arenes capture large aromatic guests such as toluene, styrene, and isopropylbenzene [25, 26]. Meanwhile, the upper and lower rims of pillararenes have many active sites that can be functionalized with kinds of substituents to increase their solubility, stability, and selectivity [27]. What’s more, the electron-rich hydrophobic cavities could accommodate diverse guests with electron-deficient and neutral guests including imidazolium cation, n-hexane, and biimidazole derivatives by non-covalent interactions, such as π-π, dipole-dipole, H-bonding, and so on [28, 29]. Different from the traditional hosts, pillararenes have highly symmetrical pillar-shaped structures and it endows them binding with guests selectively [30]. Next, pillararenes are functionalized freely on the position of the benzene rings or the phenolic rims and it makes them dissolve easily in organic solvents [31]. On the basis of the above features, pillararenes have become important platforms for the study of macrocyclic chemistry and materials science. After several years of investigation, pillararenes have been introduced into a great variety of areas, such as sensors [32], separation [33], nanomaterials [34], drug delivery [35], and so on. In GC, pillararenes which have a rigid structure, easy derivatization, and special host-guest interaction become potential candidates as new stationary phases.
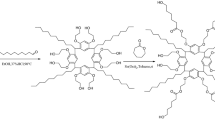
In our work, we report a novel pillar[6]arene-based material functionalized with polar ester groups (P6A-C10-OAc) with unique amphiphilic conformation for GC separations (Scheme 1). Introducing long alkyl chains can lower its melting point, thereby improving its film-forming ability. Meanwhile, the introduction of polar ester functional groups can improve its selectivity and make it more suitable for the separation of polar analytes. In this work, the P6A-C10-OAc column was investigated for its column efficiency, polarity, separation performance, and mechanism, especially for separating some challenging and important isomers. Meanwhile, the P5A-C10-OAc column and commercial HP-5, HP-35, and PEG-20M columns were employed for reference.
Experimental
Materials and equipment
All the reagents and solvents were of analytical grade and used without further purification. Temperature program: 40 ℃ (1 min) to 160 ℃ at 10 ℃/min, flow rate at 0.6 mL/min. Paraformaldehyde (POM) was obtained from Aladdin Industrial Corp. (Shanghai, China). BF3·Et2O was obtained from Alfa Aesar Co., Ltd. (Shanghai, China). Cyclohexyl chloride, 1,4-hydroquinone, 1,10-dibromodecane, and potassium carbonate were obtained from Sun Chemical Technology Co., Ltd. (Shanghai, China). Potassium acetate was obtained from Damao Chemical Reagent Co., Ltd. (Tianjin, China).
Bare fused-silica capillary columns were purchased from YongnianRuifeng Chromatogram Apparatus Co., Ltd. (Hebei, China). A commercial PEG-20M column (PEG-20M, 5 m × 0.25 mm i.d.; film thickness, 0.25 μm) was obtained from Lanzhou Atech Technologies Co., Ltd. (Gansu, China). Commercial HP-5 (5% phenyl methylpolysiloxane, 5 m × 0.25 mm i.d.; film thickness, 0.25 μm) and HP-35 (35% phenyl methylpolysiloxane, 5 m × 0.25 mm i.d.; film thickness, 0.25 μm) were obtained from Agilent Technologies Co., Ltd. (Palo Alto, CA).
An Agilent 7890A gas chromatograph including a split/splitless injector, flame ionization detector (FID), and an autosampler was used in this work to test all the analytes. All the separations were carried out under the setting GC conditions, in which the temperature of the injection port and FID detector, the split ratio, the injection volume, and the oven temperature programs for the separations are all shown in the figure captions. Thermogravimetric analysis (TGA) was used on a DTG-60AH instrument (Shimadzu, Japan). A Tensor II Fourier transform infrared spectrometer (Bruker Platinum ART) and a Bruker Biospin 400 MHz instrument (Bruker Biospin) were used to record the IR spectrum and 1HNMR spectrum, respectively.
Synthesis of the P6A-C10-OAc stationary phase
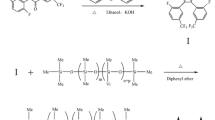
The synthetic route of P6A-C10-OAc is shown in Fig. 1. For the synthesis of C10-2Br: the compounds of 1,10-dibromodecane (10.90 g, 36.33 mmol), 1,4-hydroquinone (1.00 g, 9.08 mmol), potassium carbonate (1.26 g, 9.08 mmol), and potassium iodide (1.51 g, 9.08 mmol) were added into acetone (50 mL). The mixture was refluxed at 65 ℃ for 72 h under nitrogen, then cooled to room temperature and filtered. The filtered cake was washed with CH2Cl2, and the filtrate was evaporated under vacuum. Next, the brown residue was purified by silica-gel column chromatography with petroleum ether (b.p. 60-90 ℃)/dichloromethane (v:v = 5:1) as the eluent, and the obtained white solid was further purified by recrystallization. After drying under vacuum at 30 ℃, the white solid (22% yield) was gained. The 1H NMR and FT-IR spectrum of C10-2Br are presented in Fig. S1 and Fig. S2.
For the synthesis of P6A-C10: the mixture of C10-2Br (2.00 g, 3.65 mmol), paraformaldehyde (0.33 g, 10.94 mmol), BF3·Et2O (0.52 g, 3.65 mmol), and cyclohexyl chloride (CyC6-Cl, 30 mL) was stirred at 35 ℃ for 3 h. After the reaction is completed, deionized water (30 mL) was used to quench the reaction. Next, the organic phase was extracted by CH2Cl2 and then dried with anhydrous MgSO4. After filtering and evaporation, the white crude was purified by silica gel column chromatography with petroleum ether (b.p. 60–90 °C)/dichloromethane (v:v = 5:1 to 1:1). Finally, the P6A-C10 (10% yield) was obtained by drying under the vacuum. The 1H NMR and FT-IR spectrum of P6A-C10 are presented in Fig. S3 and Fig. S4.
For the synthesis of P6A-C10-OAc: P6A-C10 (1.00 g, 298.62 μmol) and potassium acetate (0.70 g, 7.17 mmol) were dissolved in N,N-dimethylformamide (DMF, 30 mL). Then, the solution was refluxed at 80 ℃ under nitrogen for 48 h. After completion, the solution was cooled to 25 ℃ and put into saturated brine (30 mL). Next, the yellow crude product was obtained by filtering. Finally, it was purified by silica gel column chromatography with dichloromethane/methanol (v:v = 40:1). Finally, a yellow solid (65% yield) of the P6A-C10-OAc compound was obtained after drying under vacuum. The 1H NMR and FT-IR spectrum of P6A-C10-OAc are presented in Fig. S5 and Fig. S6.
The P5A-C10-OAc was synthesized in the same procedure.
Fabrication of the P6A-C10-OAc capillary column
P6A-C10-OAc was statically coated on the inner surface of the capillary column (5 m long × 0.25 mm i.d.) [36, 37]. First of all, to roughen the inner surface of the capillary column, it was pretreated with a saturated solution of sodium chloride in methanol. After the solution already passed the column, the condition of the column was performed from 40 to 200 ℃ at 10 ℃/min and held for 3 h under nitrogen flow. Then, the pretreated column was coated with the solution of the P6A-C10-OAc stationary phase in dichloromethane (0.2%, w/v) at room temperature. Next, one end of the coated column was sealed and the other end was connected to the vacuum system to slowly remove the solvent under vacuum at 40 °C. Finally, the P6A-C10-OAc column was conditioned from 40 °C (held for 30 min) to 180 °C at 1 °C/min and held at 180 °C for 7 h under nitrogen.
Results and discussion
Characterization of the P6A-C10-OAc column
Thermogravimetric analysis (TGA) was used to measure the intrinsic thermal stability of the P6A-C10-OAc stationary phase. As illustrated in Fig. 2a, the P6A-C10-OAc stationary phase showed about 5% loss mass at 297 ℃, confirming its advantageous thermal stability as the stationary phase for GC separations [38]. To further investigate the column efficiency of the P6A-C10-OAc column, the Golay curve was determined by measuring the height equivalent to a theoretical plate (HETP) of n-dodecane at different flow rates at 120 ℃ and the results are exhibited in Fig. 2b. As shown, the minimum HETP of 0.23 mm was displayed at 0.5 mL/min corresponding to the column efficiency of 5393 plates/m. Moreover, the polarity of the P6A-C10-OAc stationary phase was characterized by McReynolds constants, which were measured by the five probe compounds containing benzene (X′), 1-butanol (Y′), 2-pentanone (Z′), 1-nitropropane (U′), and pyridine (S′) at 120 ℃ [39]. The McReynolds constants and average value of the P6A-C10-OAc columns are listed in Table 1. The average of 134 indicates its medium polarity between HP-5 and HP-35. Figure 2c presents the SEM cross-section images of the P6A-C10-OAc column, confirming its good coating with the thickness of approximately 216 nm on the capillary column.
The separation performance of the P6A-C10-OAc column
Then, to explore the selectivity and retention behaviors of the P6A-C10-OAc stationary phase, a mixture of 20 analytes with diverse polarity was utilized and three commercial columns, namely, HP-5, HP-35, and PEG-20M, were used for comparison. Figure 3 exhibits the separation of a mixture of 20 analytes containing alkanes, alcohols, esters, aldehydes, ketones, chlorobenzenes, anilines, phenols, and naphthalenes on the P6A-C10-OAc column and three commercial columns. As shown in Fig. 3, all the analytes were baseline-separated (R>1.5) and showed advantageous separation performance on the P6A-C10-OAc column, but they were co-eluted or overlapped on three commercial columns, such as 1,2,4-trimethylbenzene/octanone (peaks 2/5), dodecane/2,3-dimethylaniline (peaks 8/15), octanol/4-methylbenzaldehyde (peaks 9/10), 1,3-dibromobenzene/2,3-dimethyl phenol (peaks 12/18), and methyl decanoate/2-bromonitrobenzene (peaks 14/20) on the HP-5 column; 1,3-dibromobenzene/2,3-dimethyl phenol (peaks 12/18) on the HP-35 column; and 1,2,4-trimethylbenzene/octanone (peaks 2/5), 4-diethylbenzene/tridecane (peaks 4/11), 3-chlorobenzaldehyde/2-methylnaphthalene (peaks 13/16), and 2,3-dimethyl phenol/2-bromonitrobenzene (peaks 18/20) on the PEG-20M column. The above results proved the advantageous performance of the P6A-C10-OAc column over the commercial columns. Moreover, the elution sequences of 1,3-dibromobenzene/m-chlorobenzaldehyde (peaks 12/13, b.p. 230 ℃/213 ℃) and hexadecane/o-bromonitrobenzene (peaks 19/20, b.p. 286.79 ℃/261 ℃) were contrast to the boiling point orders on the P6A-C10-OAc column and the PEG-20M column, but were consistent with those on the HP-5 and HP-35 column, proving the stronger π-π interaction between the 3D aromatic cavity of the P6A-C10-OAc stationary phase and the polar analytes. Meanwhile, H-bonding which exists between ester groups and polar analytes could result in longer retention time and different elution sequences during the separation processes between the P6A-C10-OAc stationary phase and the analytes with polar groups, such as the following elution sequences: 1,2,4-trimethylbenzene/hexanol (peaks 2/3, b.p. 168 ℃/151 ℃), dodecane/octanol (peaks 8/9, b.p. 216.3 ℃/196 ℃), methyl decanoate/2,3-dimethylaniline (peaks 14/15, b.p. 224 ℃/221 ℃), 2-methylnaphthalene/2-bromoaniline/2,3-dmethyl phenol (peaks 16/17/18, b.p. 241 ℃/227 ℃/218 ℃). Its high separation performance is due to its unique 3D aromatic skeleton with π-rich cavity, apolar long alkyl chains and polar ester group, and the comprehensive effect of its multiple molecular interactions (π-π stacking, H-bonding, dipole-dipole, and van der Waals forces) with a wide range of analytes.
Separations of the mixture of 20 analytes of diverse types on the P6A-C10-OAc, HP-5, HP-35, and PEG-20M capillary columns. Peaks: (1) 4-dodecane, (2) 1,2,4-trimethylbenzene, (3) n-hexanol, (4) 1,4-diethylbenzene, (5) octanone, (6) 1,4-dichlorobenzene, (7) 1-bromoheptane, (8) n-dodecane, (9) n-octanol, (10) p-methylbenzaldehyde, (11) n-tridecane, (12) 1,3-dibromobenzene, (13) m-chlorobenzaldehyde, (14) methyl enanthate, (15) 2,3-dimethylaniline, (16) 2-methylnaphthalene, (17) 2-bromoaniline, (18) 2,3-dimethyl phenol, (19) n-hexadecane, (20) o-bromonitrobenzene. Temperature program: 40 ℃ (1 min) to 160 ℃ at 10℃/min, flow rate at 0.6 mL/min
Furthermore, the separation performance of the P6A-C10-OAc column was investigated by a wide variety of isomer mixtures, including alkylbenzenes, naphthalenes, halobenzenes, benzaldehydes, and phenols. As can be seen, the P6A-C10-OAc column achieved baseline resolution (R>1.5) of 14 aromatic isomers from nonpolar to polar with good peak shapes, demonstrating its ability to recognize aromatic isomers with slight differences in structure and physicochemical properties. Alkylbenzenes (Fig. 4a, b) and naphthalenes (Fig. 4c, d) without special functional groups were well separated on a column. This indicates that the P6A-C10-OAc stationary phase has high selectivity for substituted benzenes. This is attributed to the presence of slightly different π-π interactions between the three-dimensional aromatic skeleton and the single or multiple benzene rings of the analytes. In addition, the polar aromatic isomers with different substituents, such as halobenzenes (Fig. 4e–h), benzaldehydes (Fig. 4i–l), and phenols (Fig. 4m, n), were completely separated on the P6A-C10-OAc column. It is worth noting that benzaldehydes and phenols are analytes that are prone to tailing in GC separation, but they both obtain satisfactory peak shapes on the P6A-C10-OAc column [40, 41]. As can be noted, compared to the other columns, the P6A-C10-OAc column shows distinct advantages for separations of the isomers of alkylbenzenes and halogenated benzenes (diethylbenzene, ethyltoluene, dibromobenzene, chloronitrobenzene, bromonitrobenzene, bromobenzaldehyde, chlorobenzaldehyde, and nitrobenzaldehyde) in Table S1. The above findings evidenced the high separation capacity and good inertness of the P6A-C10-OAc column towards diverse types of analytes.
Separations of isomer mixtures of a diethylbenzene, b ethyltoluene, c methylnaphthalene, d dimethylnaphthalene, e dibromobenzene, f trichlorobenzene, g chloronitrobenzene, h bromonitrobenzene, i bromobenzaldehyde, j chlorobenzaldehyde, k dichlorobenzaldehyde, l nitrobenzaldehyde, m xylenol, and n hydroquinone on the P6A-C10-OAc column. GC conditions: 40 ℃ (1 min) to 160 ℃ at 10 ℃/min, flow rate at 0.6 mL/min
Xylene isomers consisted of ortho-xylene (oX), para-xylene (pX), meta-xylene (mX) and are mainly obtained from crude oil and widely applied in the petrochemical and medical industries [42]. They are important industrial raw materials for the preparation of phthalic anhydride, polyethylene terephthalate, and isophthalic acid. Separation of xylene isomers is difficult due to their similar physicochemical properties and molecular structure containing kinetic diameters, boiling points, and dipole moments [43]. The P6A-C10-OAc column was explored for its separation capability towards xylene isomers in comparison with the commercial HP-5, HP-35, and PEG-20M columns. As shown in Fig. 5a, the P6A-C10-OAc column baseline separated the xylene isomers and exhibited dramatically higher resolving capability than three commercial columns. Notably, the commercial HP-5, HP-35, and PEG-20M columns completely overlapped the pX (b.p. 138.1 ℃) and mX (b.p. 139.1 ℃). In Fig. 5b and c, the P6A-C10-OAc column completely resolved the isomers of diethylbenzene and ethyltoluene, proving its advantageous distinguishing capability for the critical pair through stronger π-π stacking interactions with alkylbenzenes due to unique 3D aromatic skeleton.
Then, the P6A-C10-OAc column was further explored on its separation capability for halobenzenes, as shown in Fig. 6a for chloronitrobenzenes, Fig. 6b for bromonitrobenzenes, Fig. 6c for bromobenzaldehydes, and Fig. 6d for chlorobenzaldehydes, respectively. The P6A-C10-OAc column baseline resolved all the halobenzenes isomers (R > 1.5) while HP-5 column coeluted p-/o-bromonitrobenzene (R = 0) and partially overlapped p-/o-chloronitrobenzene (R = 1.41), m-/p-bromonitrobenzene (R = 1.41), o-/m-bromobenzaldehyde (R = 1.21), m-/p-bromobenzaldehyde (R = 0.60), o-/m-chlorobenzaldehyde (R = 1.08), and m-/p-chlorobenzaldehyde (R = 0.87); HP-35 column partially overlapped p-/o-bromonitrobenzene (R = 1.41) and m-/p-bromobenzaldehyde (R = 1.02); and PEG-20M column completely overlapped m-/p-bromobenzaldehyde (R = 0) and m-/p-chlorobenzaldehyde (R = 0). For the P6A-C10-OAc stationary phase, additional π-π stacking and CH-π interactions from the pillararene framework may also contribute to the high resolving capability of the halobenzene isomers besides the dipole-dipole, H-bonding, and halogen bonding interactions from the polar ester groups. This result indicated the outstanding resolving ability of the P6A-C10-OAc column for the aromatic isomers varying from apolar to polar nature.
To demonstrate the effect of the cavity sizes of pillararenes on their separation performance, the separation capability of the P6A-C10-OAc column was investigated by xylene, diethylbenzene, and ethyltoluene isomers in comparison with the P5A-C10-OAc column. As shown in Fig. 7, the P6A-C10-OAc column achieved baseline resolution for all isomers, whereas the P5A-C10-OAc column completely coeluted three pairs of m-/p-xylene, o-/p-diethylbenzene, and m-/p-ethyltoluene. Therefore, the cavity sizes of pillararenes have significant impact on their separation performance, and larger P6A has better shape matching selectivity with substituted benzenes [44].
The practical application of the capillary column is also an important index to estimate its performance, we used the P6A-C10-OAc column to detect the isomer impurities in the reagent samples. Figure 8 shows the results for the determination of isomer impurities in the three reagent samples of geraniol, cis-decahydronaphthalene, and 2-chloroaniline. Table 2 summarizes their content results by the method of peak area normalization. As shown, the measured purity of the samples was consistent with their labeled values. The results proved the great potential of the P6A-C10-OAc column for the determination of the sample purities in GC.
Repeatability and durability of the P6A-C10-OAc column
The repeatability and durability of the P6A-C10-OAc column were investigated. Figure 9 shows the chromatograms of n-alkanes (n-dodecane, n-tridecane, n-tetradecane, n-pentadecane, n-hexadecane) and alcohols (1-octanol, 1-nonanol, 1-decanol, 1-undecanol, 1-dodecanol) obtained when the P6A-C10-OAc column was used for 1 month and 7 months, and after the column was subjected to 1, 25, 50, and 100 injections. There was no obvious change in the retention time of n-alkanes and alcohols, and the RSD of the retention time was less than 0.18% (Table 3), indicating the good repeatability and durability of the P6A-C10-OAc column.
The repeatability and durability of the P6A-C10-OAc column for the separation of a n-dodecane, n-tridecane, n-tetradecane, n-pentadecane, and n-cetane and b 1-octanol, 1-nonanol, 1-decanol, 1-undecanol, and 1-dodecanol. Temperature program: 40 ℃ (keep 1 min) up to 160 ℃ (keep 5 min) at 10 ℃/min and flow rate: 0.6 mL/min
Conclusion
This work describes the separation performance of the polar ester groups functionalized pillar[6]arene (P6A-C10-OAc) as the stationary phase for GC separations. As demonstrated, the P6A-C10-OAc column exhibits high-resolution performance for a wide range of analytes and isomers mainly through π-π stacking, CH-π, H-bonding, dipole-dipole, halogen-bonding, and dispersion interactions. Particularly, it achieved baseline resolution of the challenging isomers of xylenes, diethylbenzenes, ethyltoluenes, and halobenzenes and shows distinct advantages over the commercial HP-5, HP-35, and PEG-20M columns. Furthermore, we demonstrate that the cavity size of pillararenes plays an important role in the efficient separation of aromatic isomers. This work demonstrates the great potential of pillararenes as highly selective stationary phases for GC separations.
References
Latif MT, Abd Hamid HH, Ahamad F, Khan MF, Nadzir MSM, Othman M, Sahani M, Wahab MIA, Mohamad N, Uning R, Poh SC, Fadzil MF, Sentian J, Tahir NM. BTEX compositions and its potential health impacts in Malaysia. Chemosphere. 2019;237:124451. https://doi.org/10.1016/j.chemosphere.2019.124451.
Spánik I, Machynáková A. Recent applications of gas chromatography with high-resolution mass spectrometry. J Sep Sci. 2018;41(1):163–79. https://doi.org/10.1002/jssc.201701016.
Ding YJ, Alimi LO, Du J, Hua B, Dey A, Yu P. Pillar[3]trianglamines: deeper cavity triangular macrocycles for selective hexene isomer separation. Chem Sci. 2022;13(11):3244–8. https://doi.org/10.1039/d2sc00207h.
Armstrong DW, He LF, Liu YS. Examination of ionic liquids and their interaction with molecules, when used as stationary phases in gas chromatography. Anal Chem. 1999;71(17):3873–6. https://doi.org/10.1021/ac990443p.
Baltazar QQ, Leininger SK, Anderson JL. Binary ionic liquid mixtures as gas chromatography stationary phases for improving the separation selectivity of alcohols and aromatic compounds. J Chromatogr A. 2008;1182(1):119–27. https://doi.org/10.1016/j.chroma.2007.12.075.
Anderson JL, Armstrong DW. High-stability ionic liquids. A new class of stationary phases for gas chromatography. Anal Chem. 2003;75(18):4851–8. https://doi.org/10.1021/ac0345749.
Yusuf K, Badjah-Hadj-Ahmed AY, Aqel A, Alothman ZA. Fabrication of zeolitic imidazolate framework-8-methacrylate monolith composite capillary columns for fast gas chromatographic separation of small molecules. J Chromatogr A. 2015;1406:299–306. https://doi.org/10.1016/j.chroma.2015.06.026.
Chen BL, Liang CD, Yang J, Contreras DS, Clancy YL, Lobkovsky EB, Yaghi OM, Dai S. A microporous metal-organic framework for gas-chromatographic separation of alkanes. Angew Chem Int Ed. 2006;45(9):1390–3. https://doi.org/10.1002/anie.200502844.
Côté AP, Benin AI, Ockwig NW, O’Keeffe M, Matzger AJ, Yaghi OM. Porous, crystalline, covalent organic frameworks. Science. 2005;310(5751):1166–70. https://doi.org/10.1126/science.1120411.
Lohse MS, Bein T. Covalent organic frameworks: structures, synthesis, and applications. Adv Funct Mate. 2018;28(33):1705553. https://doi.org/10.1002/adfm.201705553.
Yang XH, Li CX, Qi ML, Qu LT. A graphene-based porous carbon material as a stationary phase for gas chromatographic separations. RSC Adv. 2017;7(51):32126–32. https://doi.org/10.1039/c7ra04774f.
Zarei A, Rashidi A, Tehrani MS, Azar PA. Molecular-sieve porous graphene as a steady phase of gas chromatography column for dissociation and measurement of nitrous oxide, carbon dioxide and gaseous hydrocarbons. Int J Environ Sci Technol. 2019;16(7):3049–60. https://doi.org/10.1007/s13762-018-1670-6.
Trens P, Belarbi H, Shepherd C, Gonzalez P, Ramsahye NA, Lee UH, Seo YK, Chang JS. Adsorption and separation of xylene isomers vapors onto the chromium terephthalate-based porous material MIL-101(Cr): an experimental and computational study. Microporous Mesoporous Mater. 2014;183:17–22. https://doi.org/10.1016/j.micromeso.2013.08.040.
Shinbo T, Yamaguchi T, Nishimura K, Sugiura M. Chromatogrtographic separation of racemic amino acids by use of chiral crown ether-coated reversed-phase packings. J Chromatogr. 1987;405:145–53. https://doi.org/10.1016/s0021-9673(01)81756-8.
Hyun MH, Jin JS, Lee WJ. Liquid chromatographic resolution of racemic amino acids and their derivatives on a new chiral stationary phase based on crown ether. J Chromatogr A. 1998;822:155–61. https://doi.org/10.1016/s0021-9673(98)00606-2.
Hyun MH. Preparation and application of HPLC chiral stationary phases based on (+) -(18-crown-6)-2,3,11,12-tetracarboxylic acid. J Sep Sci. 2006;29:750–61. https://doi.org/10.1002/jssc.200500431.
Juvancz Z, Alexander G, Szejtli J. Permethylated β-cyclodextrin as stationary phase in capillary gas chromatography. J High Resolut Chromatogr. 1987;10(22):105–7. https://doi.org/10.1002/jhrc.1240100214.
Konig WA, Lutz S, Wenz G. Modified cyclodextrins-novel, highly enantioselective stationary phases for gas chromatography. Angew Chem Int Ed. 1988;27(7):979–80. https://doi.org/10.1002/anie.198809791.
Armstrong D, Li WY, Pitha J. Reversing enantioselectivity in capillary gas chromatography with polar and nonpolar cyclodextrin derivative phases. Anal Chem. 1990;62(2):201–8. https://doi.org/10.1021/ac00201a023.
Zhang WF, Feng YM, Pan L, Zhang GR, Guo Y, Zhao WD, Xie ZK, Zhang SS. Silica microparticles modified with ionic liquid bonded chitosan as hydrophilic moieties for preparation of high-performance liquid chromatographic stationary phases. Microchim Acta. 2023;190(5):176. https://doi.org/10.1007/s00604-023-05755-6.
Jon SY, Selvapalam N, Oh DH, Kang JK, Kim SY, Jeon YJ, Lee JW, Kim K. Facile synthesis of cucurbit[n]uril derivatives via direct functionalization: expanding utilization of cucurbit[n]uril. J Am Chem Soc. 2003;125(34):10186–7. https://doi.org/10.1021/ja036536c.
Lee JW, Samal S, Selvapalam N, Kim HJ, Kim K. Cucurbituril homologues and derivatives: new opportunities in supramolecular chemistry. Acc Chem Res. 2003;34(8):621–30. https://doi.org/10.1021/ar020254k.
Liu SM, Ruspic C, Mukhopadhyay P, Chakrabarti S, Zavalij PY, Isaacs L. The cucurbit[n]uril family: prime components for self-sorting systems. J Am Chem Soc. 2005;127(45):15959–67. https://doi.org/10.1021/ja055013x.
Ogoshi T, Kanai S, Fujinami S, Yamagishi TA, Nakamoto Y. Para-bridged symmetrical pillar[5]arenes: their Lewis acid catalyzed synthesis and host–guest property. J Am Chem Soc. 2008;130:5022–3. https://doi.org/10.1021/ja711260m.
Hu XB, Chen ZX, Chen L, Zhang L, Hou JL, Li ZT. Pillar[n]arenes (n=8-10) with two cavities: synthesis, structures and complexing properties. Chem Commun. 2012;48(89):10999–1001. https://doi.org/10.1039/c2cc36027f.
Ogoshi T, Ueshima N, Sakakibara F, Yamagishi T, Haino T. Conversion from pillar[5]arene to pillar[6-15]arenes by ring expansion and encapsulation of C60 by pillar[n]arenes with nanosize cavities. Org Lett. 2014;16(11):2896–9. https://doi.org/10.1021/ol501039u.
Ogoshi T, Yamagishi T. Pillararenes: versatile synthetic receptors for supramolecular chemistry. Eur J Org Chem. 2013;15:2961–75. https://doi.org/10.1002/ejoc.201300079.
Arunan E, Desiraju GR, Klein RA, Sadlej J, Scheiner S, Alkorta I, Clary DC, Crabtree RH, Dannenberg JJ, Hobza P, Kjaergaard HG, Legon AC, Mennucci B, Nesbitt DJ. Definition of the hydrogen bond. Pure Appl Chem. 2011;83(8):1637–41. https://doi.org/10.1351/pac-rec-10-01-02.
Han K, Zhang YY, Li J, Yu YH, Jia XS, Li CJ. Binding mechanisms and driving forces for the selective complexation between pillar[5]arenes and neutral nitrogen heterocyclic compounds. Eur J Org Chem. 2013;2013(11):2057–60. https://doi.org/10.1002/ejoc.201201647.
Strutt NL, Zhang HC, Schneebeli ST, Stoddart JF. Functionalizing pillar[n]arenes. Acc of Chem Res. 2014;47(8):2631–42. https://doi.org/10.1021/ar500177d.
Wang KY, Jordan JH, Velmurugan K, Tian XQ, Zuo MZ, Hu XY, Wang LY. Role of functionalized pillararene architectures in supramolecular catalysis. Angew Chem Int Ed. 2020;60(17):9205–14. https://doi.org/10.1002/anie.202010150.
Yao Y, Chi XD, Zhou YJ, Huang FH. A bola-type supra-amphiphile constructed from a water-soluble pillar[5]arene and a rod–coil molecule for dual fluorescent sensing. Chem Sci. 2014;5(7):2778–82. https://doi.org/10.1039/c4sc00585f.
Jie KC, Zhou YJ, Li ER, Li ZT, Zhao R, Huang FH. Reversible iodine capture by nonporous pillar[6]arene crystals. J Am Chem Soc. 2017;139(43):15320–3. https://doi.org/10.1021/jacs.7b09850.
Zhang HC, Li C. Pillararene-functionalised graphene nanomaterials. RSC Adv. 2020;10(31):18502–11. https://doi.org/10.1039/d0ra02964e.
Duan QP, Cao Y, Li Y, Hu XY, Xiao TX, Lin C, Pan Y, Wang LY. PH-responsive supramolecular vesicles based on water-soluble pillar[6]arene and ferrocene derivative for drug delivery. J Am Chem Soc. 2013;135(28):10542–9. https://doi.org/10.1021/ja405014r.
Sun T, Chen RN, Huang QC, Ba MY, Cai ZQ, Chen HP, Qi YH, Chen H, Liu XM, Nardiello D, Quinto M. Efficient gas chromatographic separation of xylene and other aromatic isomers by using pillar [6] arene-based stationary phase. Anal Chim Acta. 2023;1251:340979. https://doi.org/10.1016/j.aca.2023.340979.
Sun T, Huang QC, Zhang W, Chen RN, Li W, Chen HP, Hu SQ, Cai ZQ. Performance and selectivity of amphiphilic pillar[5]arene as stationary phase for capillary gas chromatography. J Chromatogr A. 2022;1671:463008. https://doi.org/10.1016/j.chroma.2022.463008.
Shi YY, Qi ML. Separation performance of the copolymer and homopolymer of aliphatic polycarbonate diols as the stationary phases for capillary as chromatography. J Chromatogr A. 2021;1649:462223. https://doi.org/10.1016/j.chroma.2021.462223.
Sun T, Chen RN, Huang QC, Ba MY, Cai ZQ, Hu SQ, Liu XM, Nardiello D, Quinto M. Chromatographic separation of aromatic amine isomers: a solved issue by a new amphiphilic pillar[6]arene stationary phase. ACS Appl Mater Interfaces. 2022;14(50):56132–42. https://doi.org/10.1021/acsami.2c17889.
Poole CF, Lenca N. Gas chromatography on wall-coated open-tubular columns with ionic liquid stationary phases. J Chromatogr A. 2014;1357:87–109. https://doi.org/10.1016/j.chroma.2014.03.029.
He YR, Shi TT, Qi ML. A novel triptycene-terminated polymer used as the gas chromatographic stationary phase towards organic acidic/basic analytes and isomers. Chin Chem Lett. 2021;32(11):3372–6. https://doi.org/10.1016/j.cclet.2021.04.009.
Lue SJ, Liaw TH. Separation of xylene mixtures using polyurethane-zeolite composite membranes. Desalination. 2006;193(1–3):137–43. https://doi.org/10.1016/j.desal.2005.06.059.
Durdakova TM, Hovorka S, Hrdlicka Z, Vopicka O. Comparison of pervaporation and perstraction for the separation of p-xylene/m-xylene mixtures using PDMS and CTA membranes. Sep Purif Technol. 2021;274:118986. https://doi.org/10.1016/j.seppur.2021.118986.
Jie KC, Liu M, Zhou YJ. Near-ideal xylene selectivity in adaptive molecular pillar[n]arene crystals. J Am Chem Soc. 2018;140:6921–30. https://doi.org/10.1021/jacs.8b02621.
Funding
The work was supported by the National Natural Science Foundation of China (No. 21705072), the Colleges and Universities in Henan Province Key Science and Research Project (No. 23A150008), and the Training Project for Youth Backbone Teachers in Colleges and Universities of Luoyang Normal University.
Author information
Authors and Affiliations
Contributions
Yanli Song: investigation, methodology, formal analysis, visualization, validation, writing—original draft, writing—review and editing. Wen Li: investigation, methodology, formal analysis, validation. Mengyi Ba: methodology, visualization. Yuanyuan Zhang: validation, formal analysis. Haixin Liu: visualization, formal analysis. Xiang Xu: methodology, investigation. Haoyu Su: methodology, investigation. Zhiqiang Cai: conceptualization, methodology, supervision, resources, funding acquisition, writing—review and editing. Xianming Liu: methodology, supervision. Tao Sun: conceptualization, methodology, supervision, resources, funding acquisition, writing—review and editing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Song, Y., Li, W., Ba, M. et al. Ester-functionalized pillar[6]arene as the gas chromatographic stationary phase with high-resolution performance towards the challenging isomers of xylenes, diethylbenzenes, and ethyltoluenes. Anal Bioanal Chem 416, 1321–1335 (2024). https://doi.org/10.1007/s00216-024-05146-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-024-05146-7