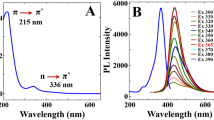
Abstract
Enzyme activity assays play a crucial role in numerous fields, including biotechnology, the food industry, and clinical diagnostics. Lipases are particularly important enzymes due to their widespread use in lipid metabolism and esterification reactions. Here, we present a pioneering method for the sensitive and selective determination of lipase activity using green carbon dots (G-CDs) for first time. G-CDs are a fascinating class of carbon nanomaterials with unique optical properties and biocompatibility, making them ideal candidates for enzyme activity assays. This approach eliminates the need for traditional fluorophores or chromogenic substrates, reducing costs, fast response time (1 min), and environmental impact with a quantum yield (QY) of 7.42%. As designed, the G-CDs fluorescent probe turn-on demonstrated a reliable linear detection range from 0 to 9 mg/mL under ideal conditions, with detection limit of 0.01 mg/mL and limit of quantification (LOQ) of 0.045 mg/mL, respectively. Furthermore, the G-CDs system was thoroughly evaluated in human serum samples, showing recoveries ranging from 100.0 to 105.0%. These findings highlight the promising applicability of the G-CDs probe for lipase detection, yielding highly favorable results.
Graphical Abstract







Similar content being viewed by others
References
Avanesov M, Loser A, Keller S, Weinrich JM, Laqmani A, Adam G, et al. Diagnosing acute pancreatitis-Clinical and radiological characterisation of patients without threefold increase of serum lipase. Eur J Radiol. 2017;95:278–85. https://doi.org/10.1016/j.ejrad.2017.08.038.
Garg PK, Singh VP. Organ Failure Due to Systemic Injury in Acute Pancreatitis. Gastroenterology. 2019;156(7):2008–23. https://doi.org/10.1053/j.gastro.2018.12.041.
Lowe AW, Luthen RE, Wong SM, Grendell JH. The level of the zymogen granule protein GP2 is elevated in a rat model for acute pancreatitis. Gastroenterology. 1994;107(6):1819–27. https://doi.org/10.1016/0016-5085(94)90826-5.
Steinberg WM, Buse JB, Ghorbani MLM, Orsted DD, Nauck MA, Committee LS, et al. Amylase, Lipase, and Acute Pancreatitis in People With Type 2 Diabetes Treated With Liraglutide: Results From the LEADER Randomized Trial. Diabetes Care. 2017;40(7):966–72. https://doi.org/10.2337/dc16-2747.
Balthazar EJJR. Acute pancreatitis: assessment of severity with clinical and CT evaluation. Radiology. 2002;223(3):603–13. https://doi.org/10.1148/radiol.2233010680.
Chen Z, Xu W, Jin L, Zha J, Tao T, Lin Y, et al. Synthesis of amine-functionalized Fe3O4@C nanoparticles for lipase immobilization. J Mater Chem A. 2014;2(43):18339–44. https://doi.org/10.1039/c4ta04117h.
Gotor-Fernández V, Brieva R, Gotor V. Lipases: Useful biocatalysts for the preparation of pharmaceuticals. J Mol Catal B Enzym. 2006;40(3–4):111–20. https://doi.org/10.1016/j.molcatb.2006.02.010.
Kalantari M, Kazemeini M, Tabandeh F, Arpanaei A. Lipase immobilisation on magnetic silica nanocomposite particles: effects of the silica structure on properties of the immobilised enzyme. J Mater Chem A. 2012;22(17):8385–93. https://doi.org/10.1039/c2jm30513e.
Lee C-H, Lin T-S, Mou C-Y. Mesoporous materials for encapsulating enzymes. Nano Today. 2009;4(2):165–79. https://doi.org/10.1016/j.nantod.2009.02.001.
Zhang W, Tang Y, Liu J, Ma Y, Jiang L, Huang W, et al. An electrochemical sensor for detecting triglyceride based on biomimetic polydopamine and gold nanocomposite. J Mater Chem B. 2014;2(48):8490–5. https://doi.org/10.1039/c4tb01439a.
Zhang W, Tang Y, Liu J, Jiang L, Huang W, Huo FW, et al. Colorimetric assay for heterogeneous-catalyzed lipase activity: enzyme-regulated gold nanoparticle aggregation. J Agric Food Chem. 2015;63(1):39–42. https://doi.org/10.1021/jf505339q.
Guan P, Liu Y, Yang B, Wu Y, Chai J, Wen G, et al. Fluorometric probe for the lipase level: Design, mechanism and biological imaging application. Talanta. 2021;225:121948. https://doi.org/10.1016/j.talanta.2020.121948.
Jette J-F, Ziomek EJAB. Determination of lipase activity by a rhodamine-triglyceride-agarose assay. Anal Biochem. 1994;219(2):256–60. https://doi.org/10.1006/abio.1994.1265.
Xie X, Li M, Tang F, Li Y, Zhang L, Jiao X, et al. Combinatorial Strategy to Identify Fluorescent Probes for Biothiol and Thiophenol Based on Diversified Pyrimidine Moieties and Their Biological Applications. Anal Chem. 2017;89(5):3015–20. https://doi.org/10.1021/acs.analchem.6b04608.
Kong F, Zhao Y, Liang Z, Liu X, Pan X, Luan D, et al. Highly Selective Fluorescent Probe for Imaging H(2)Se in Living Cells and in Vivo Based on the Disulfide Bond. Anal Chem. 2017;89(1):688–93. https://doi.org/10.1021/acs.analchem.6b03136.
Yan L, Shi H, He X, Wang K, Tang J, Chen M, et al. A versatile activatable fluorescence probing platform for cancer cells in vitro and in vivo based on self-assembled aptamer/carbon nanotube ensembles. Anal Chem. 2014;86(18):9271–7. https://doi.org/10.1021/ac5024149.
Chen D, Wu Z, Zhang Y, Li D, Wei J, Jiao T, et al. Boric acid group-functional Tb-MOF as a fluorescent and captured probe for the highly sensitive and selective determination of propyl gallate in edible oils. Food Chem. 2023;418. https://doi.org/10.1016/j.foodchem.2023.136012.
Wu Z, Wei J, Jiao T, Chen Q, Oyama M, Chen Q, et al. A lead-based room-temperature phosphorescent metal–organic framework sensor for assessing the peroxide value of edible oils. Food Chemistry. 2022;385. https://doi.org/10.1016/j.foodchem.2022.132710.
Liu H, Ding J, Zhang K, Ding L. Construction of biomass carbon dots based fluorescence sensors and their applications in chemical and biological analysis. TrAC Trends Anal Chem. 2019;118:315–37. https://doi.org/10.1016/j.trac.2019.05.051.
Chen J, Gong Z, Tang W, Row KH, Qiu H. Carbon dots in sample preparation and chromatographic separation: Recent advances and future prospects. TrAC Trends Anal Chem. 2021;134:116135. https://doi.org/10.1016/j.trac.2020.116135.
Gao P, Xie Z, Zheng M. Small nanoparticles bring big prospect: The synthesis, modification, photoluminescence and sensing applications of carbon dots. Chin Chem Lett. 2022;33(4):1659–72. https://doi.org/10.1016/j.cclet.2021.09.085.
Chen J, Yuan N, Jiang D, Lei Q, Liu B, Tang W, et al. Octadecylamine and glucose-coderived hydrophobic carbon dots-modified porous silica for chromatographic separation. Chin Chem Lett. 2021;32(11):3398–401. https://doi.org/10.1016/j.cclet.2021.04.052.
Zou G, Chen S, Liu N, Yu Y. A ratiometric fluorescent probe based on carbon dots assembly for intracellular lysosomal polarity imaging with wide range response. Chin Chem Lett. 2022;33(2):778–82. https://doi.org/10.1016/j.cclet.2021.08.076.
Zhang L, Wang Z, Wang H, Dong W, Liu Y, Hu Q, et al. Nitrogen-doped carbon dots for wash-free imaging of nucleolus orientation. Microchim Acta. 2021;188(6):183. https://doi.org/10.1007/s00604-021-04837-7.
Zhang H, Han Y, Yang Y, Chen J, Qiu H. Construction of a carbon dots/cobalt oxyhydroxide nanoflakes biosensing platform for detection of acid phosphatase. Langmuir. 2021;37(35):10529–37. https://doi.org/10.1021/acs.langmuir.1c01512.
Wu Y, Fang X, Shi J, Yao W, Wu W. Blue-to-green manipulation of carbon dots from fluorescence to ultralong room-temperature phosphorescence for high-level anti-counterfeiting. Chin Chem Lett. 2021;32(12):3907–10. https://doi.org/10.1016/j.cclet.2021.04.040.
Ma X, Lin S, Dang Y, Dai Y, Zhang X, Xia FJA, et al. Carbon dots as an “on-off-on” fluorescent probe for detection of Cu (II) ion, ascorbic acid, and acid phosphatase. Anal Bioanal Chem. 2019;411:6645–53. https://doi.org/10.1007/s00216-019-02038-z.
Liu L, Sun H, Xiao L, Yang Z-Q, Han J, Gong X, et al. Development of a highly sensitive fluorescence method for tartrazine determination in food matrices based on carbon dots. Anal Bioanal Chem. 2021;413:1485–92. https://doi.org/10.1007/s00216-020-03118-1.
Anilkumar P, Cao L, Yu JJ, Tackett KN, Wang P, Meziani MJ, et al. Crosslinked Carbon Dots as Ultra-Bright Fluorescence Probes. Small. 2013;9(4):545–51. https://doi.org/10.1002/smll.201202000.
Liu J-H, Cao L, LeCroy GE, Wang P, Meziani MJ, Dong Y, et al. Carbon “Quantum” Dots for Fluorescence Labeling of Cells. ACS Appl Mater Interfaces. 2015;7(34):19439–45. https://doi.org/10.1021/acsami.5b05665.
Guo J, Li H, Ling L, Li G, Cheng R, Lu X, et al. Green Synthesis of Carbon Dots toward Anti-Counterfeiting. ACS Sustain Chem Eng. 2019;8(3):1566–72. https://doi.org/10.1021/acssuschemeng.9b06267.
Chu S, Wang H, Du Y, Yang F, Yang L, Jiang C. Portable Smartphone Platform Integrated with a Nanoprobe-Based Fluorescent Paper Strip: Visual Monitoring of Glutathione in Human Serum for Health Prognosis. ACS Sustain Chem Eng. 2020;8(22):8175–83. https://doi.org/10.1021/acssuschemeng.0c00690.
Si M, Zhang J, He Y, Yang Z, Yan X, Liu M, et al. Synchronous and rapid preparation of lignin nanoparticles and carbon quantum dots from natural lignocellulose. Green Chemistry. 2018;20(15):3414–9. https://doi.org/10.1039/c8gc00744f.
Guo J, Lu Y, Xie AQ, Li G, Liang ZB, Wang CF, et al. Yellow-Emissive Carbon Dots with High Solid-State Photoluminescence. Adv Funct Mater. 2022;32(20):2110393. https://doi.org/10.1002/adfm.202110393.
Cai H, Liu A, Zhang M, Song J, Zeng P, Qu J, et al. Multifunctional nitrogen-doped carbon dots for HS- sensing and mitochondrial-targeted imaging. Sens Actuat B. 2022;367:132048. https://doi.org/10.1016/j.snb.2022.132048.
Yeh TF, Teng CY, Chen SJ, Teng H. Nitrogen-doped graphene oxide quantum dots as photocatalysts for overall water-splitting under visible light illumination. Adv Mater. 2014;26(20):3297–303. https://doi.org/10.1002/adma.201305299.
Iannazzo D, Pistone A, Salamo M, Galvagno S, Romeo R, Giofre SV, et al. Graphene quantum dots for cancer targeted drug delivery. Int J Pharm. 2017;518(1–2):185–92. https://doi.org/10.1016/j.ijpharm.2016.12.060.
Liu Y, Luo S, Wu P, Ma C, Wu X, Xu M, et al. Hydrothermal synthesis of green fluorescent nitrogen doped carbon dots for the detection of nitrite and multicolor cellular imaging. Anal Chim Acta. 2019;1090:133–42. https://doi.org/10.1016/j.aca.2019.09.015.
Tajik S, Dourandish Z, Zhang K, Beitollahi H, Le QV, Jang HW, et al. Carbon and graphene quantum dots: a review on syntheses, characterization, biological and sensing applications for neurotransmitter determination. RSC Adv. 2020;10(26):15406–29. https://doi.org/10.1039/d0ra00799d.
Yuan H, Li D, Liu Y, Xu X, Xiong C. Nitrogen-doped carbon dots from plant cytoplasm as selective and sensitive fluorescent probes for detecting p-nitroaniline in both aqueous and soil systems. Analyst. 2015;140(5):1428–31. https://doi.org/10.1039/c4an01869a.
Pal A, Bhakat A, Chattopadhyay A. Zinc Ion-Induced Assembly of Crystalline Carbon Dots with Excellent Supercapacitor Performance. J Phys Chem C. 2019;123(32):19421–8. https://doi.org/10.1021/acs.jpcc.9b05820.
Sun X, Bruckner C, Lei Y. One-pot and ultrafast synthesis of nitrogen and phosphorus co-doped carbon dots possessing bright dual wavelength fluorescence emission. Nanoscale. 2015;7(41):17278–82. https://doi.org/10.1039/c5nr05549k.
Yang Z, Xu M, Liu Y, He F, Gao F, Su Y, et al. Nitrogen-doped, carbon-rich, highly photoluminescent carbon dots from ammonium citrate. Nanoscale. 2014;6(3):1890–5. https://doi.org/10.1039/c3nr05380f.
Zhang C, Liu M, Li T, Liu S, Chen Q, Zhang J, et al. One-pot hydrothermal synthesis of dual-emission fluorescent carbon dots for hypochlorous acid detection. Dyes Pigm. 2020;180:108507. https://doi.org/10.1016/j.dyepig.2020.108507.
Guo J, Ye S, Li H, Song J, Qu J. One-pot synthesized nitrogen-fluorine-codoped carbon quantum dots for ClO− ions detection in water samples. Dyes Pigm. 2020;175:108178. https://doi.org/10.1016/j.dyepig.2019.108178.
Papagiannouli I, Patanen M, Blanchet V, Bozek JD, de Anda Villa M, Huttula M, et al. Depth Profiling of the Chemical Composition of Free-Standing Carbon Dots Using X-ray Photoelectron Spectroscopy. J Phys Chem C. 2018;122(26):14889–97. https://doi.org/10.1021/acs.jpcc.8b03800.
Sun S, Guan Q, Liu Y, Wei B, Yang Y, Yu Z. Highly luminescence manganese doped carbon dots. Chin Chem Lett. 2019;30(5):1051–4. https://doi.org/10.1016/j.cclet.2019.01.014.
Gao Y, Jiao Y, Zhang H, Lu W, Liu Y, Han H, et al. One-step synthesis of a dual-emitting carbon dot-based ratiometric fluorescent probe for the visual assay of Pb(2+) and PPi and development of a paper sensor. J Mater Chem B. 2019;7(36):5502–9. https://doi.org/10.1039/c9tb01203f.
Jiao Y, Meng Y, Lu W, Gao Y, Liu Y, Gong X, et al. Design of long-wavelength emission carbon dots for hypochlorous detection and cellular imaging. Talanta. 2020;219:121170. https://doi.org/10.1016/j.talanta.2020.121170.
Liu S, Liu R, Xing X, Yang C, Xu Y, Wu D. Highly photoluminescent nitrogen-rich carbon dots from melamine and citric acid for the selective detection of iron(iii) ion. RSC Adv. 2016;6(38):31884–8. https://doi.org/10.1039/c5ra26521e.
Mutuyimana FP, Liu J, Nsanzamahoro S, Na M, Chen H, Chen X. Yellow-emissive carbon dots as a fluorescent probe for chromium(VI). Microchim Acta. 2019;186(3):163. https://doi.org/10.1007/s00604-019-3284-1.
Zhang X, Chen X, Yang J, Jia H-R, Li Y-H, Chen Z, et al. Quaternized Silicon Nanoparticles with Polarity-Sensitive Fluorescence for Selectively Imaging and Killing Gram-Positive Bacteria. Adv Funct Mater. 2016;26(33):5958–70. https://doi.org/10.1002/adfm.201602185.
Pawar SP, Gore AH, Walekar LS, Anbhule PV, Patil SR, Kolekar GB. Turn-on fluorescence probe for selective and sensitive detection of d-penicillamine by CdS quantum dots in aqueous media: Application to pharmaceutical formulation. Sens Actuat B. 2015;209:911–8. https://doi.org/10.1016/j.snb.2014.12.064.
Wang G-L, Dong Y-M, Yang H-X, Li Z-J. Ultrasensitive cysteine sensing using citrate-capped CdS quantum dots. Talanta. 2011;83(3):943–7. https://doi.org/10.1016/j.talanta.2010.10.047.
Chen Y, Rosenzweig ZJAC. Luminescent CdS quantum dots as selective ion probes. Anal Chem. 2002;74(19):5132–8. https://doi.org/10.1021/ac0258251.
Lu Q, Huang T, Zhou J, Zeng Y, Wu C, Liu M, et al. Limitation-induced fluorescence enhancement of carbon nanoparticles and their application for glucose detection. Spectrochim Acta A Mol Biomol Spectrosc. 2021;244:118893. https://doi.org/10.1016/j.saa.2020.118893.
Shi J, Deng Q, Wan C, Zheng M, Huang F, Tang B. Fluorometric probing of the lipase level as acute pancreatitis biomarkers based on interfacially controlled aggregation-induced emission (AIE). Chem Sci. 2017;8(9):6188–95. https://doi.org/10.1039/c7sc02189e.
Nandu N, SalihHizir M, Roberston NM, Ozturk B, Yigit MV. Masking the Peroxidase-Like Activity of the Molybdenum Disulfide Nanozyme Enables Label-Free Lipase Detection. Chembiochem. 2019;20(14):1861–7. https://doi.org/10.1002/cbic.201800471.
Wang L, Song Y, Yao J, Wang R, Zhang N, Zou D, et al. Detection of lipase activity in rice bran with AuNPs colorimetric sensor. J Food Measure Character. 2021;15(4):3461–70. https://doi.org/10.1007/s11694-021-00916-8.
Zhang F, Du T, Jiang L, Zhu L, Tian D. A combined “AIE + ESIPT” fluorescent probe for detection of lipase activity. Bioorg Chem. 2022;128:106026. https://doi.org/10.1016/j.bioorg.2022.106026.
Luo J, Zhang H, Guan J, An B, Peng J, Zhu W, et al. Detection of lipase activity in human serum based on a ratiometric fluorescent probe. New J Chem. 2021;45(21):9561–8. https://doi.org/10.1039/d1nj01155c.
Zhang H, Wu S, Zhang L, Jiang L, Huo F, Tian D. High-resolution colorimetric detection of lipase activity based on enzyme-controlled reshaping of gold nanorods. Anal Methods. 2019;11(17):2286–91. https://doi.org/10.1039/c9ay00410f.
Shi J, Zhang S, Zheng M, Deng Q, Zheng C, Li J, et al. A novel fluorometric turn-on assay for lipase activity based on an aggregation-induced emission (AIE) luminogen. Sens Actuat B. 2017;238:765–71. https://doi.org/10.1016/j.snb.2016.07.116.
Krieg AK, Gauglitz G. An optical sensor for the detection of human pancreatic lipase. Sens Actuat B. 2014;203:663–9. https://doi.org/10.1016/j.snb.2014.07.036.
Funding
This work was supported by the Light of West China Program from Chinese Academy of Science (xbzg-zdsys-202008) and Regional Collaborative Innovation Project of Xinjiang (2020E01051), ANSO Scholarship for Young Talents in China, for the Chinese Academy of Sciences, and “Sponsored by UCAS Scholarship for International Students.
Author information
Authors and Affiliations
Contributions
Haitham Saad Al-mashriqi: visualization, methodology, investigation, data curation, validation, writing—original draft. Pascaline Sanga: software and methodology. Jia Chen: investigation, supervision, writing and revision. Xin Li: methodology. Jing Xiao: methodology. Yan Li: methodology discussion, writing and revision. Hongdeng Qiu: supervision and writing—review and editing.
Corresponding authors
Ethics declarations
Declarations
Experimental procedure on human serum was undertaken following the National Guidelines and was approved by the ethics committee of Gansu Provincial Hospital and Lanzhou Institute of Chemical Physics (Lanzhou, China).
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Al-mashriqi, H.S., Sanga, P., Chen, J. et al. Green-emitting carbon dots as a “turn on” fluorescence bio-probe for highly sensitive and selective detection of lipase in human serum. Anal Bioanal Chem 416, 971–981 (2024). https://doi.org/10.1007/s00216-023-05086-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-023-05086-8