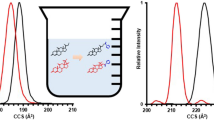
Abstract
Steroids are one of the important indicators of health and disease. However, due to the high similarity of steroid structures, there are several potential obstacles in the differentiation of steroids, especially for their isomers. Herein, we described a trapped ion mobility spectrometry-mass spectrometry (TIMS-MS) approach based on the steroid analogue adduction for isomer-specific identification of steroids. The application of dexamethasone (DEX) to form heterodimers with steroids enhanced the separation of their isomers in TIMS. Two isomer pairs including 17-hydroxyprogesterone/11-deoxycorticosterone and androsterone/epiandrosterone were successfully separated as the heterodimers with DEX by TIMS. The stability of DEX-adducted heterodimers is comparable with steroid dimers. Owing to the high separation efficiency and stability, the relative quantification of steroid isomers was demonstrated with the proposed method.
Graphical Abstract





Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Ma L, Yates SR. A review on structural elucidation of metabolites of environmental steroid hormones via liquid chromatography-mass spectrometry. Trends Anal Chem. 2018;109:142–53. https://doi.org/10.1016/j.trac.2018.10.007.
Heck AL, Crestani CC, Fernandez-Guasti A, Larco DO, Mayerhofer A, Roselli CE. Neuropeptide and steroid hormone mediators of neuroendocrine regulation. J Neuroendocrinol. 2018;30: e12599. https://doi.org/10.1111/jne.12599.
Spangenburg EE, Geiger PC, Leinwand LA, Lowe DA. Regulation of physiological and metabolic function of muscle by female sex steroids. Med Sci Sports Exerc. 2012;44:1653–62. https://doi.org/10.1249/MSS.0b013e31825871fa.
Storbeck K-H, Schiffer L, Baranowski ES, Chortis V, Prete A, Barnard L, Gilligan LC, Taylor AE, Idkowiak J, Arlt W, Shackleton CHL. Steroid metabolome analysis in disorders of adrenal steroid biosynthesis and metabolism. Endocr Rev. 2019;40(6):1605–25. https://doi.org/10.1210/er.2018-00262.
Griffiths WJ, Wang Y. Sterolomics in biology, biochemistry, medicine. Trends Anal Chem. 2019;120: 115280. https://doi.org/10.1016/j.trac.2018.10.016.
Ahn CH, Lee C, Shim J, Kong SH, Kim S-J, Kim YH, Lee KE, Shin CS, Kim JH, Choi MH. Metabolic changes in serum steroids for diagnosing and subtyping Cushing’s syndrome. J Steroid Biochem Mol Biol. 2021;210: 105856. https://doi.org/10.1016/j.jsbmb.2021.105856.
Auer MK, Krumbholz A, Bidlingmaier M, Thieme D, Reisch N. Steroid 17-hydroxyprogesterone in hair is a potential long-term biomarker of androgen control in congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Neuroendocrinology. 2020;110:938–49. https://doi.org/10.1159/000504672.
Olesti E, Boccard J, Visconti G, Gonzalez-Ruiz V, Rudaz S. From a single steroid to the steroidome: trends and analytical challenges. J Steroid Biochem Mol Biol. 2021;206: 105797. https://doi.org/10.1016/j.jsbmb.2020.105797.
Rister AL, Dodds ED. Steroid analysis by ion mobility spectrometry. Steroids. 2020;153: 108531. https://doi.org/10.1016/j.steroids.2019.108531.
Hu J, Zhang Z, Shen W-J, Azhar S. Cellular cholesterol delivery, intracellular processing and utilization for biosynthesis of steroid hormones. Nutr Metab. 2010;7:47. https://doi.org/10.1186/1743-7075-7-47.
Liao H-Y, Xiao X, Peng R, Le J, Wang H-B, Wang S-T. Rapid derivatization of phenolic and oxime hydroxyl with isonicotinoyl chloride under aqueous conditions and its application in LC-MS/MS profiling multiclass steroids. Anal Chem. 2022;94:17980–7. https://doi.org/10.1021/acs.analchem.2c04151.
Qin Q, Feng D, Hu C, Wang B, Chang M, Liu X, Yin P, Shi X, Xu G. Parallel derivatization strategy coupled with liquid chromatography-mass spectrometry for broad coverage of steroid hormones. J Chromatogr A. 2020;1614: 460709. https://doi.org/10.1016/j.chroma.2019.460709.
Liu L, Wang Z, Zhang Q, Mei Y, Li L, Liu H, Wang Z, Yang L. Ion mobility mass spectrometry for the separation and characterization of small molecules. Anal Chem. 2023;95:134–51. https://doi.org/10.1021/acs.analchem.2c02866.
Hernandez-Mesa M, Escourrou A, Monteau F, Le Bizec B, Dervilly-Pinel G. Current applications and perspectives of ion mobility spectrometry to answer chemical food safety issues. Trends Anal Chem. 2017;94:39–53. https://doi.org/10.1016/j.trac.2017.07.006.
Lapthorn C, Pullen F, Chowdhry BZ. Ion mobility spectrometry-mass spectrometry (IMS-MS) of small molecules: separating and assigning structures to ions. Mass Spectrom Rev. 2013;32:43–71. https://doi.org/10.1002/mas.21349.
Ross DH, Seguin RP, Xu L. Characterization of the impact of drug metabolism on the gas-phase structures of drugs using ion mobility-mass spectrometry. Anal Chem. 2019;91:14498–507. https://doi.org/10.1021/acs.analchem.9b03292.
Chouinard CD, Cruzeiro VWD, Roitberg AE, Yost RA. Experimental and theoretical investigation of sodiated multimers of steroid epimers with ion mobility-mass spectrometry. J Am Soc Mass Spectrom. 2017;28:323–31. https://doi.org/10.1007/s13361-016-1525-7.
Hernandez-Mesa M, D’Atri V, Barknowitz G, Fanuel M, Pezzatti J, Dreolin N, Ropartz D, Monteau F, Vigneau E, Rudaz S, Stead S, Rogniaux H, Guillarme D, Dervilly G, Le Bizec B. Interlaboratory and interplatform study of steroids collision cross section by traveling wave ion mobility spectrometry. Anal Chem. 2020;92:5013–22. https://doi.org/10.1021/acs.analchem.9b05247.
Mackay CLL, Soltwisch J, Heijs B, Smith KW, Cruickshank FL, Nyhuis A, Dreisewerd K, Cobice D. Spatial distribution of isobaric androgens in target tissues using chemical derivatization and MALDI-2 on a trapped ion mobility quadrupole time-of-flight instrument. RSC Adv. 2021;11:33916–25. https://doi.org/10.1039/d1ra06086d.
Ahonen L, Fasciotti M, afGennas GB, Kotiaho T, Daroda RJ, Eberlin M, Kostiainen R. Separation of steroid isomers by ion mobility mass spectrometry. J Chromatogr A. 2013;1310:133–7. https://doi.org/10.1016/j.chroma.2013.08.056.
Rister AL, Martin TL, Dodds ED. Application of group I metal adduction to the separation of steroids by traveling wave ion mobility spectrometry. J Am Soc Mass Spectrom. 2019;30:248–55. https://doi.org/10.1007/s13361-018-2085-9.
Rister AL, Martin TL, Dodds ED. Formation of multimeric steroid metal adducts and implications for isomer mixture separation by traveling wave ion mobility spectrometry. J Mass Spectrom. 2019;54(5):429–36. https://doi.org/10.1002/jms.4350.
White PC. Neonatal screening for congenital adrenal hyperplasia. Nat Rev Endocrinol. 2009;5:490–8. https://doi.org/10.1038/nrendo.2009.148.
Kaabia Z, Laparre J, Cesbron N, Le Bizec B, Dervilly-Pinel G. Comprehensive steroid profiling by liquid chromatography coupled to high it resolution mass spectrometry. J Steroid Biochem Mol. 2018;183:106–15. https://doi.org/10.1016/j.jsbmb.2018.06.003.
Dodds JN, May JC, McLean JA. Correlating resolving power, resolution, and collision cross section: unifying cross-platform assessment of separation efficiency in ion mobility spectrometry. Anal Chem. 2017;89(22):12176–84. https://doi.org/10.1021/acs.analchem.7b02827.
Wang H, Wu F, Xu F, Liu Y, Ding C-F. Identification of bi-2-naphthol and its phosphate derivatives complexed with cyclodextrin and metal ions using trapped ion mobility spectrometry. Anal Chem. 2021;93:15096–104. https://doi.org/10.1021/acs.analchem.1c03378.
Fouque DJD, Lartia R, Maroto A, Memboeuf A. Quantification of intramolecular click chemistry modified synthetic peptide isomers in mixtures using tandem mass spectrometry and the survival yield technique. Anal Bioanal Chem. 2018;410(23):5765–77. https://doi.org/10.1007/s00216-018-1258-5.
Funding
This work was financially supported by the National Natural Science Foundation of China (22204085) and the Zhejiang Provincial Natural Science Foundation of China under Grant No. LQ23B050005.
Author information
Authors and Affiliations
Contributions
Yang Li: data curation; visualization; investigation; methodology; writing original draft. Yujiao Qin: methodology; investigation. Songchang Wei: methodology; investigation. Ling Ling: resources; funding acquisition; supervision; conceptualization; writing—review and editing. Chuan-Fan Ding: supervision; resources; funding acquisition; writing—review and editing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, Y., Qin, Y., Wei, S. et al. Differentiation of steroid isomers by steroid analogues adducted trapped ion mobility spectrometry-mass spectrometry. Anal Bioanal Chem 416, 313–319 (2024). https://doi.org/10.1007/s00216-023-05019-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-023-05019-5