Abstract
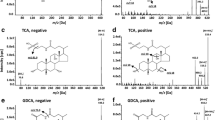
Bile acids (BAs) are a complex class of metabolites that have been described as specific biomarkers of gut microbiota activity. The development of analytical methods allowing the quantification of an ample spectrum of BAs in different biological matrices is needed to enable a wider implementation of BAs as complementary measures in studies investigating the functional role of the gut microbiota. This work presents results from the validation of a targeted ultra-high performance liquid chromatography-tandem mass spectrometry (UHPLC-MS/MS) method for the determination of 28 BAs and six sulfated BAs, covering primary, secondary, and conjugated BAs. The analysis of 73 urine and 20 feces samples was used to test the applicability of the method. Concentrations of BAs in human urine and murine feces were reported, ranging from 0.5 to 50 nmol/g creatinine and from 0.012 to 332 nmol/g, respectively. Seventy-nine percent of BAs present in human urine samples corresponded to secondary conjugated BAs, while 69% of BAs present in murine feces corresponded to primary conjugated BAs. Glycocholic acid sulfate (GCA-S) was the most abundant BA in human urine samples, while taurolithocholic acid was the lowest concentrated compound detected. In murine feces, the most abundant BAs were α-murocholic, deoxycholic, dehydrocholic, and β-murocholic acids, while GCA-S was the lowest concentrated BA. The presented method is a non-invasive approach for the simultaneous assessment of BAs and sulfated BAs in urine and feces samples, and the results will serve as a knowledge base for future translational studies focusing on the role of the microbiota in health.
Graphical Abstract




Similar content being viewed by others
References
Vaz FM, Ferdinandusse S. Bile acid analysis in human disorders of bile acid biosynthesis. Mol Aspects Med. 2017;56:10–24. https://doi.org/10.1016/j.mam.2017.03.003.
Chassaing B, Gewirtz AT. Gut microbiome and metabolism, in: Physiology of the Gastrointestinal Tract, Elsevier. 2018: pp. 775–793. https://doi.org/10.1016/B978-0-12-809954-4.00035-9.
Ridlon JM, Kang D-J, Hylemon PB. Bile salt biotransformations by human intestinal bacteria. J Lipid Res. 2006;47:241–59. https://doi.org/10.1194/jlr.R500013-JLR200.
Vasavan T, Ferraro E, Ibrahim E, Dixon P, Gorelik J, Williamson C. Heart and bile acids – Clinical consequences of altered bile acid metabolism. Biochimica et Biophysica Acta (BBA) - Mol Basis Dis. 2018:1864;1345–55. https://doi.org/10.1016/j.bbadis.2017.12.039.
Li S, Li C, Wang W. Bile acid signaling in renal water regulation. Am J Physiol Renal Physiol. 2019;317:F73–6. https://doi.org/10.1152/ajprenal.00563.2018.
Straniero S, Laskar A, Savva C, Härdfeldt J, Angelin B, Rudling M. Of mice and men: murine bile acids explain species differences in the regulation of bile acid and cholesterol metabolism. J Lipid Res. 2020;61:480–91. https://doi.org/10.1194/jlr.RA119000307.
Lin Q, Tan X, Wang W, Zeng W, Gui L, Su M, Liu C, Jia W, Xu L, Lan K. Species differences of bile acid redox metabolism: tertiary oxidation of deoxycholate is conserved in preclinical animals. Drug Metab Dispos. 2020;48:499–507. https://doi.org/10.1124/dmd.120.090464.
Russell DW. The enzymes, regulation, and genetics of bile acid synthesis. Annu Rev Biochem. 2003;72:137–74. https://doi.org/10.1146/annurev.biochem.72.121801.161712.
Dawson PA. Bile formation and the enterohepatic circulation, in: Physiology of the Gastrointestinal Tract, Elsevier. 2018: pp. 931–956. https://doi.org/10.1016/B978-0-12-809954-4.00041-4.
Alnouti Y. Bile acid sulfation: a pathway of bile acid elimination and detoxification. Toxicol Sci. 2009;108:225–46. https://doi.org/10.1093/toxsci/kfn268.
Barbier O, Trottier J, Kaeding J, Caron P, Verreault M. Lipid-activated transcription factors control bile acid glucuronidation. Mol Cell Biochem. 2009;326:3–8. https://doi.org/10.1007/s11010-008-0001-5.
Durník R, Šindlerová L, Babica P, Jurček O. Bile acids transporters of enterohepatic circulation for targeted drug delivery. Molecules. 2022;27:2961. https://doi.org/10.3390/molecules27092961.
Zwicker BL, Agellon LB. Transport and biological activities of bile acids. Int J Biochem Cell Biol. 2013;45:1389–98. https://doi.org/10.1016/j.biocel.2013.04.012.
Bathena SPR, Thakare R, Gautam N, Mukherjee S, Olivera M, Meza J, Alnouti Y. Urinary bile acids as biomarkers for liver diseases I. Stability of the Baseline Profile in Healthy Subjects. Toxicol Sci. 2015;143:296–307. https://doi.org/10.1093/toxsci/kfu227.
Dosedělová V, Itterheimová P, Kubáň P. Analysis of bile acids in human biological samples by microcolumn separation techniques: a review. Electrophoresis. 2021;42:68–85. https://doi.org/10.1002/elps.202000139.
Zheng X, Huang F, Zhao A, Lei S, Zhang Y, Xie G, Chen T, Qu C, Rajani C, Dong B, Li D, Jia W. Bile acid is a significant host factor shaping the gut microbiome of diet-induced obese mice. BMC Biol. 2017;15:120. https://doi.org/10.1186/s12915-017-0462-7.
Steiner C, von Eckardstein A, Rentsch KM. Quantification of the 15 major human bile acids and their precursor 7α-hydroxy-4-cholesten-3-one in serum by liquid chromatography-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2010;878:2870–80. https://doi.org/10.1016/j.jchromb.2010.08.045.
Perwaiz S, Tuchweber B, Mignault D, Gilat T, Yousef IM. Determination of bile acids in biological fluids by liquid chromatography-electrospray tandem mass spectrometry. J Lipid Res. 2001;42:114–9. https://doi.org/10.1016/S0022-2275(20)32342-7.
García-Cañaveras JC, Donato MT, Castell JV, Lahoz A. Targeted profiling of circulating and hepatic bile acids in human, mouse, and rat using a UPLC-MRM-MS-validated method. J Lipid Res. 2012;53:2231–41. https://doi.org/10.1194/jlr.D028803.
Humbert L, Maubert MA, Wolf C, Duboc H, Mahé M, Farabos D, Seksik P, Mallet JM, Trugnan G, Masliah J, Rainteau D. Bile acid profiling in human biological samples: comparison of extraction procedures and application to normal and cholestatic patients. J Chromatogr B. 2012;899:135–45. https://doi.org/10.1016/j.jchromb.2012.05.015.
Sarafian MH, Lewis MR, Pechlivanis A, Ralphs S, McPhail MJW, Patel VC, Dumas M-E, Holmes E, Nicholson JK. Bile acid profiling and quantification in biofluids using ultra-performance liquid chromatography tandem mass spectrometry. Anal Chem. 2015;87:9662–70. https://doi.org/10.1021/acs.analchem.5b01556.
Ushijima K, Kimura A, Inokuchi T, Yamato Y, Maeda K, Yamashita Y, Nakashima E, Kato H. Placental transport of bile acids: analysis of bile acids in maternal serum and urine, umbilical cord blood, and amniotic fluid., Kurume. Med J. 2001;48:87–91. https://doi.org/10.2739/kurumemedj.48.87.
Kumar BS, Chung BC, Lee Y-J, Yi HJ, Lee B-H, Jung BH. Gas chromatography-mass spectrometry-based simultaneous quantitative analytical method for urinary oxysterols and bile acids in rats. Anal Biochem. 2011;408:242–52. https://doi.org/10.1016/j.ab.2010.09.031.
Matysik S, Schmitz G. Application of gas chromatography–triple quadrupole mass spectrometry to the determination of sterol components in biological samples in consideration of the ionization mode. Biochimie. 2013;95:489–95. https://doi.org/10.1016/j.biochi.2012.09.015.
Food and Drug Administration (FDA). US Department of Health and Human Services. Bioanalytical method validation. Guidance for industry. 2018.
Committee for Medicinal Products for Human Use (CHMP). Guideline on validation of bioanalytical methods. 2022.
Takahashi S, Fukami T, Masuo Y, Brocker CN, Xie C, Krausz KW, Wolf CR, Henderson CJ, Gonzalez FJ. Cyp2c70 is responsible for the species difference in bile acid metabolism between mice and humans. J Lipid Res. 2016;57:2130–7. https://doi.org/10.1194/jlr.M071183.
Acknowledgements
The authors would like to thank all study participants, as well as the nurses and medical staff involved, for their effort and commitment.
Funding
This work was funded by the European Union’s Horizon 2020 Research and Innovation Programme through the Nutrishield project (https://nutrishield-project.eu/) [Grant Agreement No 818110] and the Carlos III Health Institute (Ministry of Science and Innovation, Spain) and co-funded by the European Union [grant numbers CD19/00176, and CPII21/00003], grant Despega IATA from IATA-CSIC, the Spanish Ministry of Science and Innovation [grant number PID2020-119536RB-I00], and MCIN/AEI/https://doi.org/10.13039/501100011033 and by ERDF A way of making Europe, by the “European Union” [grant number PID2021-125573OB-I00].
Author information
Authors and Affiliations
Contributions
Conceptualization: Julia Kuligowski and Guillermo Quintás. Methodology: Victoria Ramos-Garcia. Formal analysis: Victoria Ramos-Garcia, Guillermo Quintás. Data curation: Victoria Ramos-Garcia, Guillermo Quintás. Writing—original draft preparation: Victoria Ramos-Garcia, Clara Bullich-Vilarrubias, Marina Romaní-Pérez, Yolanda Sanz, Angelica Nobili, Marika Falcone, Marina Di Stefano. Writing—review and editing: all authors. Supervision: Julia Kuligowski, Isabel Ten-Doménech, and Máximo Vento. Project administration: Julia Kuligowski. Funding acquisition: Julia Kuligowski. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval
During the enrolment phase, pediatricians informed the parents about the purpose of this study, the lack of reported risks related to the collection of samples, the effort required to take part in this study, and their right to deny or withdraw their consent at any time. Parents accepted to participate in the study and signed an informed consent form. The study was approved by the Institutional Ethical Committee of the IRCCS San Raffaele Scientific Institute (Protocol: NUTRI-T1D, 2019). The experimental procedure using animals was in accordance with European Union 2010/63/UE and Spanish RD53/2013 guidelines and approved by the ethics committee of the University of Valencia (Animal Production Section, SCSIE, University of Valencia, Spain) and authorized by Dirección General de Agricultura, Ganadería y Pesca (Generalitat Valenciana) (approval ID 2021/VSC/PEA/0273).
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ramos-Garcia, V., Ten-Doménech, I., Vento, M. et al. Fast profiling of primary, secondary, conjugated, and sulfated bile acids in human urine and murine feces samples. Anal Bioanal Chem 415, 4961–4971 (2023). https://doi.org/10.1007/s00216-023-04802-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-023-04802-8