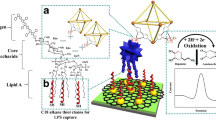
Abstract
Metal–organic frameworks (MOFs) are hybrid materials constructed by the linkage between an inorganic secondary building unit and an organic linker. A number of MOFs are luminescent in nature and can be structurally tuned for desirable geometry, surface functionality, and porosity. Luminescent MOFs have been endorsed for various biosensing applications. Lectins and carbohydrates have been used for the development of simple and convenient biosensing and bioimaging tools. Lectins are mostly present on the surface of microorganisms where they aid in pathogenesis. Due to this, they can be potential targets for a microbial biosensor. The present study, for the first time, explores the usage of a carbohydrate-conjugated FeMOF (Glyco-MOF) bioprobe for the selective determination of Pseudomonas aeruginosa and Escherichia coli. NH2-MIL-53(Fe) MOF was synthesized via a room temperature protocol and separately conjugated with galactose and mannose sugars via glutaraldehyde chemistry. The synthesized bioprobe is validated for structural integrity, luminescent nature, stability, and analyte assay. Electron microscopy studies validated the unhindered MOF’s morphology and structural integrity, after bioconjugation. The synthesized bioprobes were able to detect P. aeruginosa and E. coli up to respective detection limits of 202 and 8 CFU/mL, respectively. The bioprobes are selective even in co-presence of possible interferants as well as being environmentally stable.
Graphical Abstract







Similar content being viewed by others
References
Potrzebowski MJ, Kaźmierski S, Kassassir H, Miksa B. Phosphorus-31 NMR spectroscopy of condensed matter. Annu Rep NMR Spectrosc. 2010;70:35–114.
James SL. Metal-organic frameworks. Chem Soc Rev. 2003;32(5):276–88.
Mehta J, et al. Application of an enzyme encapsulated metal-organic framework composite for convenient sensing and degradation of methyl parathion. Sens Actuators, B Chem. 2019;290:267–74.
Bhardwaj N, Bhardwaj S, Mehta J, Kim K-H, Deep A. Highly sensitive detection of dipicolinic acid with a water-dispersible terbium-metal organic framework. Biosens Bioelectron. 2016;86:799–804.
Kaur H, et al. Luminescent metal-organic frameworks and their composites: potential future materials for organic light emitting displays. Coord Chem Rev. 2019;401: 213077.
Sumby CJ. A thin film opening. Nat Chem. 2016;8(4):294–6.
Ben T, et al. Targeted synthesis of a porous aromatic framework with high stability and exceptionally high surface area. Angew Chem. 2009;121(50):9621–4.
Bhardwaj N, Bhardwaj SK, Mehta J, Kim K-H, Deep A. MOF–bacteriophage biosensor for highly sensitive and specific detection of Staphylococcus aureus. ACS Appl Mater Interfaces. 2017;9(39):33589–98.
Cui Y, Yue Y, Qian G, Chen B. Luminescent functional metal–organic frameworks. Chem Rev. 2012;112(2):1126–62.
Allendorf MD, Bauer CA, Bhakta R, Houk R. Luminescent metal–organic frameworks. Chem Soc Rev. 2009;38(5):1330–52.
Heine J, Müller-Buschbaum K. Engineering metal-based luminescence in coordination polymers and metal–organic frameworks. Chem Soc Rev. 2013;42(24):9232–42.
Mehta J, Dhaka S, Paul AK, Dayananda S, Deep A. Organophosphate hydrolase conjugated UiO-66-NH2 MOF based highly sensitive optical detection of methyl parathion. Environ Res. 2019;174:46–53.
Zhu G, et al. A metal-organic zeolitic framework with immobilized urease for use in a tapered optical fiber urea biosensor. Microchim Acta. 2020;187(1):1–9.
Abdolmohammad-Zadeh H, Ahmadian F. A fluorescent biosensor based on graphene quantum dots/zirconium-based metal-organic framework nanocomposite as a peroxidase mimic for cholesterol monitoring in human serum. Microchem J. 2021;164: 106001.
Liu H, et al. A dual-signal electroluminescence aptasensor based on hollow Cu/Co-MOF-luminol and g-C3N4 for simultaneous detection of acetamiprid and malathion. Sens Actuators, B Chem. 2021;331: 129412.
Afzalinia A, Mirzaee M. Ultrasensitive fluorescent miRNA biosensor based on a “sandwich” oligonucleotide hybridization and fluorescence resonance energy transfer process using an Ln (III)-MOF and Ag nanoparticles for early cancer diagnosis: application of central composite design. ACS Appl Mater Interfaces. 2020;12(14):16076–87.
Cohen SM. Modifying MOFs: new chemistry, new materials. Chem Sci. 2010;1(1):32–6.
Hao J-N, Yan B. A water-stable lanthanide-functionalized MOF as a highly selective and sensitive fluorescent probe for Cd 2+. Chem Commun. 2015;51(36):7737–40.
Bhardwaj N, Bhardwaj SK, Bhatt D, Tuteja SK, Kim K-H, Deep A. Highly sensitive optical biosensing of Staphylococcus aureus with an antibody/metal–organic framework bioconjugate. Anal Methods. 2019;11(7):917–23.
Silva PM, Lima AL, Silva BV, Coelho LC, Dutra RF, Correia MT. Cratylia mollis lectin nanoelectrode for differential diagnostic of prostate cancer and benign prostatic hyperplasia based on label-free detection. Biosens Bioelectron. 2016;85:171–7.
Yang Y, Yu M, Yan T-T, Zhao Z-H, Sha Y-L, Li Z-J. Characterization of multivalent lactose quantum dots and its application in carbohydrate–protein interactions study and cell imaging. Bioorg Med Chem. 2010;18(14):5234–40.
Bertók T, Katrlík J, Gemeiner P, Tkac J. Electrochemical lectin based biosensors as a label-free tool in glycomics. Microchim Acta. 2013;180(1–2):1–13.
Ghazarian H, Idoni B, Oppenheimer SB. A glycobiology review: carbohydrates, lectins and implications in cancer therapeutics. Acta Histochem. 2011;113(3):236–47.
Becer CR. The glycopolymer code: synthesis of glycopolymers and multivalent carbohydrate–lectin interactions. Macromol Rapid Commun. 2012;33(9):742–52.
Hushegyi A, Pihíková D, Bertok T, Adam V, Kizek R, Tkac J. Ultrasensitive detection of influenza viruses with a glycan-based impedimetric biosensor. Biosens Bioelectron. 2016;79:644–9.
Yang H, Zhou H, Hao H, Gong Q, Nie K. Detection of Escherichia coli with a label-free impedimetric biosensor based on lectin functionalized mixed self-assembled monolayer. Sens Actuators, B Chem. 2016;229:297–304.
Xi F, Gao J, Wang J, Wang Z. Discrimination and detection of bacteria with a label-free impedimetric biosensor based on self-assembled lectin monolayer. J Electroanal Chem. 2011;656(1–2):252–7.
Yang H, Jie X, Wang L, Zhang Y, Wang M, Wei W. An array consisting of glycosylated quantum dots conjugated to MoS 2 nanosheets for fluorometric identification and quantitation of lectins and bacteria. Microchim Acta. 2018;185(11):512.
Kaushal S, Priyadarshi N, Pinnaka AK, Soni S, Deep A, Singhal NK. Glycoconjugates coated gold nanorods based novel biosensor for optical detection and photothermal ablation of food borne bacteria. Sens Actuators, B Chem. 2019;289:207–15.
Wu M, Li X. Klebsiella pneumoniae and Pseudomonas aeruginosa. Molecular medical microbiology: Elsevier; 2015. p. 1547–64.
Zhang X, Xie G, Gou D, Luo P, Yao Y, Chen H. A novel enzyme-free electrochemical biosensor for rapid detection of Pseudomonas aeruginosa based on high catalytic Cu-ZrMOF and conductive Super P. Biosens Bioelectron. 2019;142: 111486.
Bhardwaj N, Bhardwaj SK, Bhatt D, Lim DK, Kim K-H, Deep A. Optical detection of waterborne pathogens using nanomaterials. TrAC, Trends Anal Chem. 2019;113:280–300.
Bhardwaj N, Bhardwaj SK, Nayak MK, Mehta J, Kim K-H, Deep A. Fluorescent nanobiosensors for the targeted detection of foodborne bacteria. TrAC, Trends Anal Chem. 2017;97:120–35.
Shah N, Naseby D. Validation of constitutively expressed bioluminescent Pseudomonas aeruginosa as a rapid microbiological quantification tool. Biosens Bioelectron. 2015;68:447–53.
Jia F, et al. A magnetic relaxation switch aptasensor for the rapid detection of Pseudomonas aeruginosa using superparamagnetic nanoparticles. Microchim Acta. 2017;184(5):1539–45.
Gomes TA, Elias WP, Scaletsky IC, Guth BE, Rodrigues JF, Piazza RM, et al. Diarrheagenic Escherichia coli. Braz J Microbiol. 2016;47:3–30.
Allocati N, Masulli M, Alexeyev MF, Di Ilio C. Escherichia coli in Europe: an overview. Int J Environ Res Public Health. 2013;10(12):6235–54.
Schröer F, et al. Lectin and E. coli binding to carbohydrate-functionalized oligo (ethylene glycol)-based microgels: effect of elastic modulus, crosslinker and carbohydrate density. Molecules. 2021;26(2):263.
Sauer MM, et al. Binding of the bacterial adhesin FimH to its natural, multivalent high-mannose type glycan targets. J Am Chem Soc. 2018;141(2):936–44.
Wen L, Zhou L, Zhang B, Meng X, Qu H, Li D. Multifunctional amino-decorated metal–organic frameworks: nonlinear-optic, ferroelectric, fluorescence sensing and photocatalytic properties. J Mater Chem. 2012;22(42):22603–9.
Bhardwaj N, Bhardwaj SK, Mehta J, Nayak MK. Deep A Bacteriophage conjugated IRMOF-3 as a novel opto-sensor for S. arlettae. New J Chem. 2016;40(9):8068–73.
Li M, Zheng Z, Zheng Y, Cui C, Li C, Li Z. Controlled growth of metal–organic framework on upconversion nanocrystals for NIR-enhanced photocatalysis. ACS Appl Mater Interfaces. 2017;9(3):2899–905.
Van Tran T, et al. Application of Fe-based metal-organic framework and its pyrolysis products for sulfonamide treatment. Environ Sci Pollut Res. 2019;26(27):28106–26.
Geng N, et al. Insights into the novel application of Fe-MOFs in ultrasound-assisted heterogeneous Fenton system: efficiency, kinetics and mechanism. Ultrason Sonochem. 2021;72: 105411.
Gupta A, Garg M, Singh S, Deep A, Sharma AL. Highly sensitive optical detection of Escherichia coli using terbium-based metal–organic framework. ACS Appl Mater Interfaces. 2020;12(42):48198–205.
Sharon N, Lis H. History of lectins: from hemagglutinins to biological recognition molecules. Glycobiology. 2004;14(11):53R-62R.
Hollett G, et al. Quantum ensembles of silicon nanoparticles: discrimination of static and dynamic photoluminescence quenching processes. The Journal of Physical Chemistry C. 2019;123(29):17976–86.
Cacita N, Nikolaou S. Studying the interaction between trinuclear ruthenium complexes and human serum albumin by means of fluorescence quenching. J Lumin. 2016;169:115–20.
Acknowledgements
The authors are thankful to the Department of Biotechnology (DBT), India, for project grant IC-12025(11)/2020-ICD-DBT. We thank the Director, CSIR-CSIO, Chandigarh, for providing necessary infrastructure facilities.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bhatt, D., Singh, S., Singhal, N. et al. Glyco-conjugated metal–organic framework biosensor for fluorescent detection of bacteria. Anal Bioanal Chem 415, 659–667 (2023). https://doi.org/10.1007/s00216-022-04455-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-022-04455-z