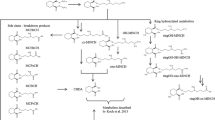
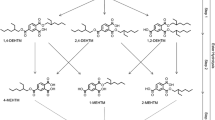
Abstract
Triclosan (TCS) is an antibacterial and antifungal compound found in many hygiene products, including toothpaste, soap, and detergents. However, this molecule can act as an endocrine disruptor and can induce harmful effects on human health and the environment. In this study, triclosan was biotransformed in vitro using human and rat liver fractions, to evaluate oxidative metabolism, the formation of reactive metabolites via the detection of GSH adducts, as well as glucuronide and sulfate conjugates using liquid chromatography coupled to high-resolution tandem mass spectrometry (LC-HRMS/MS). A deuterated analog of triclosan was also employed for better structural elucidation of specific metabolic sites. Several GSH adducts were found, either via oxidative metabolism of triclosan or its cleavage product, 2,4-dichlorophenol. We also detected glucuronide and sulfated conjugates of triclosan and its cleaved product. This study was aimed at understanding the routes of detoxification of this xenobiotic, as well as investigating any potential pathways related to additional toxicity via reactive metabolite formation.

Graphical abstract





Similar content being viewed by others
References
Zafar AB, Butler RC, Reese DJ, Gaydos LA, Mennonna PA. Use of 0.3% triclosan (Bacti-Stat) to eradicate an outbreak of methicillin-resistant Staphylococcus aureus in a neonatal nursery. Am J Infect Control. 1995;23:200–8.
Villalain J, Mateo CR, Aranda FJ, Shapiro S, Micol V. Membranotropic effects of the antibacterial agent triclosan. Arch Biochem Biophys. 2001;390:128–36.
Guillen J, Bernabeu A, Shapiro S, Villalain J. Location and orientation of triclosan in phospholipid model membranes. Eur Biophys J. 2004;33:448–53.
Heath RJ, Li J, Roland GE, Rock CO. Inhibition of the Staphylococcus aureus NADPH-dependent enoyl-acyl carrier protein reductase by triclosan and hexachlorophene. J Biol Chem. 2000;275:4654–9.
Stewart MJ, Parikh S, Xiao G, Tonge PJ, Kisker C. Structural basis and mechanism of enoyl reductase inhibition by triclosan. J Mol Biol. 1999;290:859–65.
Sandborgh Englund G, Adolfsson-Erici M, Odham G, Ekstrand J. Pharmacokinetics of triclosan following oral ingestion in humans. J Toxicol Environ Health. 2006;69:1861–73.
Lin YJ. Buccal absorption of triclosan following topical mouthrinse application. Am J Dent. 2000;13:215–7.
Bagley DM, Lin YJ. Clinical evidence for the lack of triclosan accumulation from daily use in dentifrices. Am J Dent. 2000;13:148–52.
Chedgzoy P, Winckle G, Heard CM. Triclosan: release from transdermal adhesive formulations and in vitro permeation across human epidermal membranes. Int J Pharm. 2002;235:229–36.
Moss T, Howes D, Williams FM. Percutaneous penetration and dermal metabolism of triclosan (2,4, 4′-trichloro-2′ hydroxydiphenyl ether). Food Chem Toxicol. 2000;38:361–70.
Siddiqui WH, Buttar HS. Pharmacokinetics of triclosan in rat after intravenous and intravaginal administration. J Environ Pathol Toxicol. 1979;2:861–71.
Ishibashi H, Matsumura N, Hirano M, Matsuoka M, Shiratsuchi H, Ishibashi Y, et al. Effects of triclosan on the early life stages and reproductionof medaka Oryzias latipes and induction of hepatic vitellogenin. Aquat Toxicol. 2004;67:167–79.
Cao X-Y, Hua X, Xiong J-W, Zhu W-T, Zhang J, Chen L. Impact of Triclosan on Female Reproduction through Reducing Thyroid Hormones to Suppress Hypothalamic Kisspeptin Neurons in Mice. Front Mol Neurosci. 2018 (January 19), https://doi.org/10.3389/fnmol.2018.00006
Yueh M-F, Taniguchi K, Chen S, Evans RM, Hammock BD, Karin M, Tukey RH. The commonly used antimicrobial additive triclosan is a liver tumor promoter. PNAS 2014;111(48):17200-17205
Hess Wilson JK, Knudse K-E. Endocrine disrupting compounds and prostate cancer. Cancer Lett. 2006;241:1–12.
Mink PJ, Adami HO, Trichopoulos D, Britton NL, Mandel JS. Pesticides and prostate cancer: a review of epidemiologic studies with specific agricultural exposure information. Eur J Cancer Prev. 2008;17:97–110.
Seaman PF, Ochs D, Day MJ. Comment on: Triclosan resistance in methicillin-resistant Staphylococcus aureus expressed as small colony variants: a novel mode of evasion of susceptibility to antibiotics. J Antimicrob Chemother. 2007;60:175–6.
Tkachenko O, Shepard J, Aris VM, Joy A. A triclosan-ciprofloxacin cross-resistant mutant strain of Staphylococcus aureus displays an alteration in the expression of several cell membrane structural and functional genes. Res Microbial. 2007;158:651–8.
Zuckerbraun HL, Babich H, May R, Sinensky MC. Triclosan: cytotoxicity, mode of action, and induction of apoptosis in human gingival cells in vitro. Eur J Oral Sci. 1998;106:628–36.
Zhang Y, Liu M, Liu J, Wang X, Wang C, Ai W, et al. Combined toxicity of triclosan, 2, 4-dichlorophenol and 2, 4, 6-trichlorophenol to zebrafish (Danio rerio). Environ Toxicol Pharmacol. 2018;57:9–18.
Gaume B, Bourgougnon N, Auzoux-Bordenave S, Roig B, Le Bot B, Bedoux G. In vitro effects of triclosan and methyl-triclosan on the marine gastropod Haliotis tuberculata. Comp Biochem Physiol C Toxicol Pharmacol. 2012;156(2):87–94.
Weatherly LM, Gosse JA. Triclosan exposure, transformation, and human health effects. J Toxicol Environ Health B. 2017;20(8):447–69.
Wu JL, Liu J, Cai Z. Determination of triclosan metabolites by using in-source fragmentation from high-performance liquid chromatography/ negative atmospheric pressure chemical ionization ion trap mass spectrometry. Rapid Commun Mass Spectrom. 2010;24(13):1828–34.
Provencher G, Bérubé R, Dumas P, Bienvenu JF, Gaudreau E, Bélanger P, et al. Determination of bisphenol A, triclosan and their metabolites in human urine using isotope-dilution liquid chromatography–tandem mass spectrometry. J Chromatogr A. 2014;1348:97–104.
Yin J, Wei L, Shi Y, Zhang J, Wu Q, Shao B. Chinese population exposure to triclosan and triclocarban as measured via human urine and nails. Environ Geochem Health. 2016;38(5):1125–35.
Fang J-L, Vanlandingham M, da Costa G-G, Beland F-A. Absorption and metabolism of triclosan after application to the skin of B 6 C 3 F 1 mice. Environ Toxicol. 2016;31(5):609–23.
Zhu M, Ma L, Zhang H, Humphreys WG. Detection and structural characterization of glutathione-trapped reactive metabolites using liquid chromatography high-resolution mass spectrometry and mass defect filtering. Anal Chem. 2007;79(21):8333–834.
Zhao SX, Dalvie DK, Kelly JM, Soglia JR, Frederick KS, Smith EB, et al. NADPH-dependent covalent binding of [3H] paroxetine to human liver microsomes and S-9 fractions: identification of an electrophilic quinone metabolite of paroxetine. Chem Res Toxicol. 2007;20(11):1649–57.
Zheng J, Ma L, Xin B, Olah T, Humphreys WG, Zhu M. Screening and identification of GSH-trapped reactive metabolites using hybrid triple quadruple linear ion trap mass spectrometry. Chem Res Toxicol. 2007;20(5):757–66.
Wang LQ, Falany CN, James MO. Triclosan as a substrate and inhibitor of 3-phosphoadenosine 5_-phosphosulfotransferase and UDP-glucuronosyl transferase in human liver fractions. Drug Metab Dispos. 2004;32:1162–9.
Lee SK, Kim DH, Yoo HH. Comparative metabolism of sildenafil in liver microsomes of different species by using LC/MS-based multivariate analysis. J Chromatogr B. 2011;879(28):3005–11.
Tang J, Usmani KA, Hodgson E, Rose RL. In vitro metabolism of fipronil by human and rat cytochrome P450 and its interactions with testosterone and diazepam. Chem-Biol Int. 2004;147(3):319–29.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Guesmi, A., Sleno, L. In vitro metabolism of triclosan studied by liquid chromatography–high-resolution tandem mass spectrometry. Anal Bioanal Chem 412, 335–342 (2020). https://doi.org/10.1007/s00216-019-02239-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-019-02239-6