Abstract
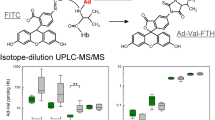
4,4′-Methylene diphenyl diisocyanate (MDI) is one of the most important isocyanates in the industrial production of polyurethane and other MDI-based synthetics. Because of its high reactivity, it is known as a sensitizing agent, caused by protein adducts. Analysis of MDI is routinely done by determination of the nonspecific 4,4′-methylenedianiline as a marker for MDI exposure in urine and blood. Since several publications have reported specific adducts of MDI and albumin or hemoglobin, more information about their existence in humans is necessary. Specific adducts of MDI and hemoglobin were only reported in rats after high-dose MDI inhalation. The aim of this investigation was to detect the hemoglobin adduct 5-isopropyl-3-[4-(4-aminobenzyl)phenyl]hydantoin (ABP-Val-Hyd) in human blood for the first time. We found values up to 5.2 ng ABP-Val-Hyd/g globin (16 pmol/g) in blood samples of workers exposed to MDI. Because there was no information available about possible amounts of this specific MDI marker, the analytical method focused on optimal sensitivity and selectivity. Using gas chromatography–high-resolution mass spectrometry with negative chemical ionization, we achieved a detection limit of 0.02 ng ABP-Val-Hyd/g globin (0.062 pmol/g). The robustness of the method was confirmed by relative standard deviations between 3.0 and 9.8 %. Combined with a linear detection range up to 10 ng ABP-Val-Hyd/g globin (31 pmol/g), the enhanced precision parameter demonstrates that the method described is optimized for screening studies of the human population.





Similar content being viewed by others
References
European Chemicals Agency (2008) Data on manufacture, import, export, uses and releases of 4,4’-diaminodiphenylmethane as well as information on potential alternatives to its use. http://echa.europa.eu/documents/10162/13640/tech_rep_mda_en.pdf. Accessed 11 Mar 2013)
Wisneswski AV, Xu L, Robinson E, Liu J, Redlich CA, Herrick CA (2011) Immune sensitization to methylene diphenyl diisocyanate (MDI) resulting from skin exposure: Albumin as a carrier protein connecting skin exposure to subsequent respiratory responses. J Occup Med Toxicol 6:6
Pauluhn J, Leng G (2003) Concentration-dependence of biomarkers of exposure of methylenediphenyl-diisocyanate following acute inhalation exposure of rats. Toxicology 185:35–48
Sabbioni G (1992) Hemoglobin binding of monocyclic aromatic amines: Molecular dosimetry and quantitative structure activity relationships fort the N-oxidation. Chem Biol Interact 81:91–117
Reisser M, Schmidt BF, Brown WE (2002) Synthesis, characterization and solvolysis of mono- and bis-S-(glutathionyl) adducts of methylene-bis-(phenylisocyanate) (MDI). Chem Res Toxicol 15:1235–1241
Birner G, Neumann HG (1988) Biomonitoring of aromatic amines II: hemoglobin binding of some monocyclic aromatic amines. Arch Toxicol 62:110–115
Bolognesi C, Baur X, Marczynski B, Norppa H, Sepai O, Sabbioni G (2001) Carcinogenic risk of toluene diisocyanate and 4,4’-methylenediphenyl diisocyanate: epidemiological and experimental evidence. Crit Rev Toxicol 31(6):737–772
Vock EH, Vamvakas S, Gahlmann R, Lutz WK (1998) Investigation of the induction of DNA double-strand breaks by methylenediphenyl-4,4‘-diisocyanate in cultured human lung epithelial cells. Toxicol Sci 46:83–89
Sennbro CJ, Lindh CH, Tinnerberg H, Gustavsson C, Littorin M, Welinder H, Jönsson BAG (2003) Development, validation and characterization of an analytical method for the quantification of hydrolysable urinary metabolites and plasma protein adducts of 2,4- and 2,6-toluene diisocyanate, 1,5-naphthalene diisocyanate and 4,4’-methylenediphenyl diisocyanate. Biomarkers 8(3–4):204–217
Gledhill A, Wake A, Hext P, Leibold E, Shiotsuka R (2005) Absorption, distribution, metabolism and excretion of an inhalation dose of [14C] 4,4′-methylenediphenyl diisocyanate in the male rat. Xenobiotica 35(3):273–292
Robert A, Ducos P, Francin JM, Marsan P (2007) Biological monitoring of workers exposed to 4,4’-methylenediphenyl diisocyanate (MDI) in 19 French polyurethane industries. Int Arch Occup Environ Health 80(5):412–422
Tiljander A, Skarping G, Dalene M (1989) Chromatographic determination of amines in biological fluids with special reference to the biological monitoring of isocyanates and amines III: determination of 4,4’-methylenedianiline in hydrolysed human urine using derivatization and capillary gas chromatography with selected ion monitoring. J Chromatogr 479:145–152
Cocker J, Brown LC, Wilson HK (1988) A GC/MS method for the determination of 4,4’-diaminodiphenylmethane and substituted analogues in urine. J Anal Toxicol 12:9–14
Johannesson G, Sennbro CJ, Willix P, Lindh CH, Jönsson BAG (2004) Identification and characterisation of adducts between serum albumin and 4,4‘-methylenediphenyl diisocyanate (MDI) in human plasma. Arch Toxicol 78:378–383
Sepai O, Schütze D, Heinrich U, Hoymann HG, Henschler D, Sabbioni G (1995) Hemoglobin adducts and urine metabolites of 4,4‘-methylenedianiline after 4,4‘-methylenediphenyl diisocyanate exposure of rats. Chem Biol Interact 97:185–198
Schütze D, Sepai O, Lewalter J, Miksche L, Henschler D, Sabbioni G (1995) Biomonitoring of workers exposed to 4,4‘-methylenedianiline or 4,4‘-methylenediphenyl diisocyanate. Carcinogenesis 16(3):573–582
Sabbioni G, Beyerbach A (1995) Determination of hemoglobin adducts of arylamines in humans. J Chromatogr B 667:75–83
Bailey E, Brooks AG, Bird I, Farmer PB, Street B (1990) Monitoring exposure to 4,4’-methylenedianiline by the gas chromatography-mass spectrometry determination of adducts to hemoglobin. Anal Biochem 190:175–181
Sabbioni G, Hartley R, Henschler D, Höllrigl-Rosta A, Koeber R, Schneider S (2000) Isocyanate-specific hemoglobin adduct in rats exposed to 4,4‘-methylenediphenyl diisocyanate. Chem Res Toxicol 13:82–89
Kumar A, Dongari N, Sabbioni G (2009) New isocyanate-specific hemoglobin adduct in rats exposed to 4,4’-methylenediphenyl-diisocyanate. Chem Res Toxicol 22(12):1975–1983
Sabbioni G, Dongari N, Kumar A (2010) Determination of a new biomarker in subjects exposed to 4,4’-methylenediphenyl diisocyanate. Biomarkers 15(6):508–515
Stark GR (1965) Reactions of cyanate with functional groups of proteins. III. Reactions with amino and carboxyl groups. Biochemistry 4(6):1030–1036
Kwan JTC, Carr EC, Bending MR, Barron JL (1990) Determination of carbamylated hemoglobin by high performance liquid chromatography. Clin Chem 36(4):607–610
Nigen AM, Bass BD, Manning JM (1976) Reactivity of cyanate with valine-1 (α) of hemoglobin. J Biol Chem 251(23):7638–7643
van Sittert NJ (1997) N-2-Cyanoethylevaline, N-2-hydroxyethylvaline, N-methylvaline (as evidence of exposure to acrylonitrile, ethylene oxide as well as methylating agents). In: Angerer J, Schaller KH (eds) Analyses of hazardous substances in biological materials, vol 5. VCH, Weinheim
Lewalter J, Biedermann P (1993) Aromatic amines (aniline, o-, m- and p-toluidine, 4-chloro-o-toluidine, 2,4-toluylenediamine and 2,6-toluylenediamine, 4-aminodiphenyl, 4,4’-diaminodiphenylmethane). In: Angerer J, Schaller KH (eds) Analyses of harzardous substances in biological materials, vol 4. VCH, Weinheim
Lewalter J, Gries W (2000) Haemoglobin adducts of aromatic amines: aniline, o-, m- and p-toluidine, o-anisidine, p-chloroaniline, α- and β-naphthylamine, 4-aminodiphenyl, benzidine, 4,4’-diaminodiphenylmethane, 3,3’-dichlorobenzidine. In: Angerer J, Schaller KH (eds) Analyses of harzardous substances in biological materials, vol 5. Wiley-VCH, Weinheim
Acknowledgments
The development of this method of analyzing MDI-specific hemoglobin adducts found in the human body was funded by the German chemicals industry. The method was developed during an ongoing 10-year project on human biomonitoring. The project is a cooperation agreed in 2010 between the Federal Ministry for the Environment, Nature Conservation and Nuclear Safety (BMU) and the Verband der chemischen Industrie (German Chemical Industry Association—VCI) and is administered by the Federal Environment Agency (UBA). Experts from public authorities, industry, and science support the project in selecting substances and developing methods. We would like to thank the International Isocyanate Institute for sponsoring the syntheses of the calibration standards.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gries, W., Leng, G. Analytical determination of specific 4,4′-methylene diphenyl diisocyanate hemoglobin adducts in human blood. Anal Bioanal Chem 405, 7205–7213 (2013). https://doi.org/10.1007/s00216-013-7171-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-013-7171-z