Abstract
Heme and heme degradation products play critical roles in numerous biological phenomena which until now have only been partially understood. One reason for this is the very low concentrations at which free heme, its complexes and the partly unstable degradation products occur in living cells. Therefore, powerful and specific detection methods are needed. In this contribution, the potential of nondestructive Raman spectroscopy for the detection, quantification and discrimination of heme and heme degradation products is investigated. Resonance Raman spectroscopy using different excitation wavelengths (413, 476, 532, and 752 nm) is employed to estimate the limit of detection for hemin, myoglobin, biliverdin, and bilirubin. Concentrations in the low micromolar range (down to 3 μmol/L) could be reliably detected when utilizing the resonance enhancement effect. Furthermore, a systematic study on the surface-enhanced Raman spectroscopy (SERS) detection of hemin in the presence of other cellular components, such as the highly similar cytochrome c, DNA, and the important antioxidant glutathione, is presented. A microfluidic device was used to reproducibly create a segmented flow of aqueous droplets and oil compartments. Those aqueous droplets acted as model chambers where the analytes have to compete for the colloid. With the help of statistical analysis, it was possible to detect and differentiate the pure substances as well as the binary mixtures and gain insights into their interaction.

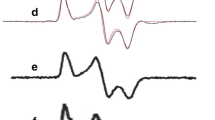
Resonance Raman spectrum of met-myoglobin and Raman intensity - concentration - calibration plot.







Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.References
Furuyama K, Kaneko K, Vargas PD (2007) Heme as a magnificent molecule with multiple missions: heme determines its own fate and governs cellular homeostasis. Tohoku J Exp Med 213(1):1–16
Ryter SW, Tyrrell RM (2000) The Heme synthesis and degradation pathways: role in oxidant sensitivity—heme oxygenase has both pro- and antioxidant properties. Free Radic Biol Med 28(2):289–309. doi:10.1016/s0891-5849(99)00223-3
Hou S, Reynolds MF, Horrigan F, Heinemann SH, Hoshi T (2006) Reversible binding of heme to proteins in cellular signal transduction. Accounts Chem Res 39:918–924
Kumar S, Bandyopadhyay U (2005) Free heme toxicity and its detoxification systems in human. Toxicol Lett 157(3):175–188
Smith WE (2008) Practical understanding and use of surface enhanced Raman scattering/surface enhanced resonance Raman scattering in chemical and biological analysis. Chem Soc Rev 37(5):955–964. doi:10.1039/b708841h
Efremov EV, Ariese F, Gooijer C (2008) Achievements in resonance Raman spectroscopy review of a technique with a distinct analytical chemistry potential. Anal Chim Acta 606(2):119–134. doi:10.1016/j.aca.2007.11.006
Pahlow S, März A, Seise B, Hartmann K, Freitag I, Kämmer E, Böhme R, Deckert V, Weber K, Cialla D, Popp J (2012) Bioanalytical application of surface- and tip-enhanced Raman spectroscopy. Eng Life Sci 12:131–143
Hering K, Cialla D, Ackermann K, Doerfer T, Moeller R, Schneidewind H, Mattheis R, Fritzsche W, Rösch P, Popp J (2008) SERS: a versatile tool in chemical and biochemical diagnostics. Anal Bioanal Chem 390:113–124
Kho KW, Fu CY, Dinish US, Olivo M (2011) Clinical SERS: are we there yet? J Biophotonics 4:667–684
Nie S, Emory SR (1997) Probing single molecules and single nanoparticles by surface-enhanced Raman scattering. Science 275:1102–1106
Kneipp J, Kneipp H, Kneipp K (2008) SERS—a single-molecule and nanoscale tool for bioanalytics. Chem Soc Rev 37(5):1052–1060. doi:10.1039/b708459p
Ackermann KR, Henkel T, Popp J (2007) Quantitative online detection of low-concentrated drugs via a SERS microfluidic system. Chem Phys Chem 8:2665–2670
Strehle KR, Cialla D, Rösch P, Henkel T, Köhler M, Popp J (2007) A reproducible surface-enhanced Raman spectroscopy approach. Online SERS measurements in a segmented microfluidic system. Anal Chem 79:1542–1547
März A, Ackermann KR, Malsch D, Bocklitz T, Henkel T, Popp J (2009) Towards a quantitative SERS approach—online monitoring of analytes in a microfluidic system with isotope-edited internal standards. J Biophotonics 2:232–242
Henkel T, Bermig T, Kielpinski M, Grodrian A, Metze J, Kohler JM (2004) Chip modules for generation and manipulation of fluid segments for micro serial flow processes. Chem Eng J 101(1–3):439–445. doi:10.1016/j.cej.2004.01.021
März A, Bocklitz T, Popp J (2011) Online-calibration for reliable and robust lab-on-a-chip surface enhanced Raman spectroscopy measurement in a liquid/liquid segmented flow. Anal Chem 83(21):8337–8340. doi:10.1021/ac2015799
Lee PC, Meisel D (1982) Adsorption and surface-enhanced Raman of dyes on silver and gold sols. J Phys Chem 86:3391–3395
R Development Core Team R (2011) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Ryan CG, Clayton E, Griffin WL, Sie SH, Cousens DR (1988) SNIP, a statistics-sensitive background treatment for the quantitative analysis of PIXE spectra in geosience aplications. Nucl Instrum Meth Phys Res B34(34):396–402
Brown SB, Dean TC, Jones P (1970) Aggregation of ferrihaems. Dimerization and protolytic equilibria of protoferrihaem and deuteroferrihaem in aqueous solution. Biochem J 117:733–739
Kuzelova K, Mrhalova M, Hrkal Z (1997) Kinetics of heme interaction with heme-binding proteins: the effect of heme aggregation state. Biochimica Et Biophysica Acta-Gen Subj 1336:497–501
Wood BR, Langford SJ, Cooke BM, Lim J, Glenister FK, Duriska M, Unthank JK, McNaughton D (2004) Resonance Raman spectroscopy reveals new insight into the electronic structure of beta-hematin and malaria pigment. J Am Chem Soc 126(30):9233–9239. doi:10.1021/ja038691x
Wu CH, Xu BH, Zhao JA, Jiang QB, Wei FD, Jiang H, Wang XM, Yan H (2010) Ferrocene-substituted dithio-o-carborane isomers: influence on the native conformation of myoglobin protein. Chem Eur J 16(29):8914–8922. doi:10.1002/chem.201000605
Krois D, Lehner H (1993) Helically fixed chiral bilirubins and biliverdins—a new insight into the conformational, associative and dynamic features of linear tetrapyrrols. J Chem Soc-Perkin Trans 2(7):1351–1360. doi:10.1039/p29930001351
Russell MP, Coulthurst SJ, Moore JN, Hester RE (1995) Resonance Raman study of the chromophore specific vibrational and electronic spectra of bilirubin. J Chem Soc-Faraday Trans 91(12):1751–1757. doi:10.1039/ft9959101751
Hu SZ, Smith KM, Spiro TG (1996) Assignment of protoheme resonance Raman spectrum by heme labeling in myoglobin. J Am Chem Soc 118(50):12638–12646
Abe M, Kitagawa T, Kyogoku Y (1978) Resonance Raman spectra of octaethylporphyrinato–Ni(II) and meso–deuterated and 15N substituted derivatives. I. Observation and assignments of nonfundamental Raman lines. J Chem Phys 69:4516–4526
Hu JM, Moigno D, Kiefer W, Ma JS, Chen QQ, Wang CQ, Feng HT, Shen JK, Niu F, Gu YH (2000) Fourier-transform Raman and infrared spectroscopic analysis of novel biliverdin compounds. Spectrochimic Acta Mol Biomol Spectros 56(12):2365–2372
Hu JM, Liang EJ, Duschek F, Kiefer W (1997) Resonance Raman spectroscopic study of free brilirubin and brilirubin complexes with copper(II), silver(I) and gold(III). Spectrochim Acta Mol Biomol Spectros 53(9):1431–1438. doi:10.1016/s0584-8539(96)01839-9
Sanchez LA, Spiro TG (1985) Surface-enhanced raman-spectroscopy as a monitor of iron(III) protoporphyrin reduction at a silver electrode in aqueous and acetonitrile solutions—vibronic resonance enhancement amplified by surface enhancement. J Phys Chem 89(5):763–768. doi:10.1021/j100251a009
Yeo BS, Maedler S, Schmid T, Zhang WH, Zenobi R (2008) Tip-enhanced Raman spectroscopy can see more: the case of cytochrome c. J Phys Chem C 112:4867–4873
Cavalu S, Cinta-Pinzaru S, Leopold N, Kiefer W (2001) Raman and surface enhanced Raman spectroscopy of 2,2,5,5-tetramethyl-3-pyrrolin-1-yloxy-3-carboxamide labeled proteins: bovine serum albumin and cytochrome c. Biopolymers 62:341–348
Delfino I, Bizzarri AR, Cannistraro S (2005) Single-molecule detection of yeast cytochrome c by surface-enhanced Raman spectroscopy. Biophys Chem 113(1):41–51. doi:10.1016/j.bpc.2004.07.006
Feng F, Zhi G, Jia HS, Cheng L, Tian YT, Li XJ (2009) SERS detection of low-concentration adenine by a patterned silver structure immersion plated on a silicon nanoporous pillar array. Nanotechnology 20(29):doi:29550110.1088/0957-4484/20/29/295501
Sari MA, Battioni JP, Dupre D, Mansuy D, Lepecq JB (1990) Interaction of cationic porphyrins with DNA: importance of the number and position of the charges and minimum structural requirements for intercalation. Biochemistry 29:4205–4215
Qu F, Zhu ZW, Li NQ (2000) Electrochemical studies of the hemin-DNA interaction. Electroanalysis 12(11):831–835
Li SD, Su YD, Li M, Zou CG (2006) Hemin-mediated hemolysis in erythrocytes: effects of ascorbic acid and glutathione. Acta Biochim Et Biophys Sin 38(1):63–69. doi:10.1111/j.1745-7270.2006.00127.x
Toader AM, Diaconu C, Volanschi E (2007) Spectroelectrochemical study of the hemin–glutathione interaction in the absence and presence of surfactants. ECS Trans 3(29):155–166
Atamna H, Ginsburg H (1995) Heme degradation in the presence of glutathione—a proposed mechanism to account for the high-levels of nonheme iron found in the membranes of hemoglobinopathic red-blood-cells. J Biol Chem 270(42):24876–24883
Shviro Y, Shaklai N (1987) Glutathione as a scavenger of free hemin—a mechanism of preventing red-cell membrane damage. Biochem Pharmacol 36(22):3801–3807
Huang GG, Hossain MK, Han XX, Ozaki Y (2009) A novel reversed reporting agent method for surface-enhanced Raman scattering; highly sensitive detection of glutathione in aqueous solutions. Analyst 134:2468–2474
Larsson M, Lindgren J (2005) Analysis of glutathione and immunoglobulin G inside chromatographic beads using surface-enhanced Raman scattering spectroscopy. J Raman Spectros 36:394–399
Sheng C, Zhao H, Gu FK, Yang HF (2009) Effect of Pb2+ on l-glutathione monolayers on a silver surface investigated by surface-enhanced Raman scattering spectroscopy. J Raman Spectros 40:1274–1278
Picquart M, Grajcar L, Baron MH, Abedinzadeh Z (1999) Vibrational spectroscopic study of glutathione complexation in aqueous solutions. Biospectroscopy 5:328–337
Neugebauer U, Heinemann SH, Schmitt M, Popp J (2011) Combination of patch clamp and Raman spectroscopy for single-cell analysis. Anal Chem 83(1):344–350. doi:10.1021/ac1024667
Acknowledgments
Financial support by TMWFK PE114-1, DFG (FOR 1738 - Po563/17-1), BMBF (FKZ 01EO1002), European Union (EFRE), the Free State of Thuringia and Thüringer Aufbaubank (2008FE9112) are highly acknowledged. Mathias Köhler and Stefan Bode are highly acknowledged for assisting the resonance Raman measurements, Claudia Beleites for fruitful discussions and Gisela Persch for preparing the colloids.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in the special paper collection Optical Biochemical and Chemical Sensors with guest editor Laura M. Lechuga.
Rights and permissions
About this article
Cite this article
Neugebauer, U., März, A., Henkel, T. et al. Spectroscopic detection and quantification of heme and heme degradation products. Anal Bioanal Chem 404, 2819–2829 (2012). https://doi.org/10.1007/s00216-012-6288-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-012-6288-9