Abstract
Peroxisomes produce reactive oxygen species which may participate in biotransformations of innate biomolecules and xenobiotics. Isolating functional peroxisomes with low levels of contaminants would be a useful tool to investigate biotransformations occurring in these organelles that are usually confounded with biotransformations occurring in other co-isolated organelles. Here, we immunoisolate peroxisomes and demonstrate that the impurity level after isolation is low and that peroxisomes retain their biological activity. In this method, an antibody targeting a 70-kDa peroxisomal membrane protein was immobilized to silanized magnetic iron oxide beads (1–4 μm in diameter) coated with Protein A. Peroxisomes from L6 rat myoblast homogenates were magnetically captured, washed, and then analyzed for subcellular composition using enzymatic assays. Based on the ratio of peroxisomal to lysosomal activity, the retained fraction is 70-fold enriched relative to the unretained fraction. Similarly, the ratio of peroxisomal activity to mitochondrial content suggests that the retained fraction is >30-fold enriched relative to the unretained fraction. H2O2 production from the β-oxidation of palmitoyl-CoA demonstrated that the isolated peroxisomal fraction was biologically active. Capillary electrophoresis with laser-induced fluorescence detection (CE-LIF) analysis confirmed that the immunopurified fractions were capable of transforming the anticancer drug doxorubicin and the fatty acid analog, BODIPY 500/510 C1C12. Besides its use to investigate peroxisome biotransformations in health and disease, the combination of magnetic immunoisolation with CE-LIF could be widely applicable to investigate subcellular-specific biotransformations of xenobiotics occurring at immunoisolated subcellular compartments.

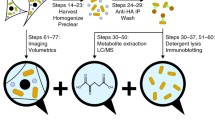
Peroxisomes are immunoisolated using Protein A-coated magnetic beads. The isolated peroxisomes are highly enriched relative to other contaminating organelles. Capillary electrophoretic analysis shows that isolated peroxisomes can biotransform doxorubicin, a common anti-cancer drug.




Similar content being viewed by others
References
Schrader M, Fahimi HD (2008) The peroxisome: still a mysterious organelle. Histochem Cell Biol 129(4):421–440
Schrader M, Fahimi HD (2004) Mammalian peroxisomes and reactive oxygen species. Histochem Cell Biol 122(4):383–393
Wanders RJ, Vreken P, Ferdinandusse S, Jansen GA, Waterham HR, van Roermund CW, Van Grunsven EG (2001) Peroxisomal fatty acid alpha- and beta-oxidation in humans: enzymology, peroxisomal metabolite transporters and peroxisomal diseases. Biochem Soc Trans 29(Pt 2):250–267
Wanders RJ, Waterham HR (2006) Biochemistry of mammalian peroxisomes revisited. Annu Rev Biochem 75:295–332
Wanders RJ, Waterham HR (2006) Peroxisomal disorders: the single peroxisomal enzyme deficiencies. Biochim Biophys Acta 1763(12):1707–1720
Masters CJ (1998) On the role of the peroxisome in the metabolism of drugs and xenobiotics. Biochem Pharmacol 56(6):667–673
Graham JM (2001) Isolation of peroxisomes from tissues and cells by differential and density gradient centrifugation. Curr Protoc Cell Biol Chapter 3:Unit 3.5
Volkl A, Fahimi HD (1985) Isolation and characterization of peroxisomes from the liver of normal untreated rats. Eur J Biochem 149(2):257–265
Luers GH, Hartig R, Mohr H, Hausmann M, Fahimi HD, Cremer C, Volkl A (1998) Immuno-isolation of highly purified peroxisomes using magnetic beads and continuous immunomagnetic sorting. Electrophoresis 19(7):1205–1210
Kikuchi M, Hatano N, Yokota S, Shimozawa N, Imanaka T, Taniguchi H (2004) Proteomic analysis of rat liver peroxisome: presence of peroxisome-specific isozyme of Lon protease. J Biol Chem 279(1):421–428
Blum RH, Carter SK (1974) Adriamycin. A new anticancer drug with significant clinical activity. Ann Intern Med 80(2):249–259
Menna P, Salvatorelli E, Minotti G (2010) Anthracycline degradation in cardiomyocytes: a journey to oxidative survival. Chem Res Toxicol 23(1):6–10
Taatjes DJ, Gaudiano G, Resing K, Koch TH (1997) Redox pathway leading to the alkylation of DNA by the anthracycline, antitumor drugs adriamycin and daunomycin. J Med Chem 40(8):1276–1286
Reinhoud NJ, Tjaden UR, Irth H, van der Greef J (1992) Bioanalysis of some anthracyclines in human plasma by capillary electrophoresis with laser-induced fluorescence detection. J Chromatogr 574(2):327–334
Simeon N, Chatelut E, Canal P, Nertz M, Couderc F (1999) Anthracycline analysis by capillary electrophoresis. Application to the analysis of daunorubicine in Kaposi sarcoma tumor. J Chromatogr A 853(1–2):449–454
Anderson AB, Gergen J, Arriaga EA (2002) Detection of doxorubicin and metabolites in cell extracts and in single cells by capillary electrophoresis with laser-induced fluorescence detection. J Chromatogr B 769(1):97–106
Anderson AB, Arriaga EA (2004) Subcellular metabolite profiles of the parent CCRF-CEM and the derived CEM/C2 cell lines after treatment with doxorubicin. J Chromatogr B 808(2):295–302
Eder AR, Arriaga EA (2006) Capillary electrophoresis monitors enhancement in subcellular reactive oxygen species production upon treatment with doxorubicin. Chem Res Toxicol 19(9):1151–1159
Anderson AB, Xiong G, Arriaga EA (2004) Doxorubicin accumulation in individually electrophoresed organelles. J Am Chem Soc 126(30):9168–9169
Chen Y, Walsh RJ, Arriaga EA (2005) Selective determination of the doxorubicin content of individual acidic organelles in impure subcellular fractions. Anal Chem 77(8):2281–2287
Lu L, Wang Y (2008) Immunoprecipitation alert: DNA binding proteins directly bind to protein A/G without any antibody as the bridge. Cell Cycle 7(3):417–418
Storrie B, Madden EA (1990) Isolation of subcellular organelles. Methods Enzymol 182:203–225
Barrett AJ, Heath MF (1977) Lysosomal enzymes. In: Dingle JT (ed) Lysosomes: a laboratory handbook. Elsevier, Amsterdam
Petit JM, Maftah A, Ratinaud MH, Julien R (1992) 10N-nonyl acridine orange interacts with cardiolipin and allows the quantification of this phospholipid in isolated mitochondria. Eur J Biochem 209(1):267–273
Mannaerts GP, Debeer LJ (1982) Mitochondrial and peroxisomal beta-oxidation of fatty acids in rat liver. Ann N Y Acad Sci 386:30–39
Mueller S, Weber A, Fritz R, Mutze S, Rost D, Walczak H, Volkl A, Stremmel W (2002) Sensitive and real-time determination of H2O2 release from intact peroxisomes. Biochem J 363(Pt 3):483–491
Wanders RJ, van Roermund CW, de Vries CT, van den Bosch H, Schrakamp G, Tager JM, Schram AW, Schutgens RB (1986) Peroxisomal beta-oxidation of palmitoyl-CoA in human liver homogenates and its deficiency in the cerebro-hepato-renal (Zellweger) syndrome. Clin Chim Acta 159(1):1–10
Hows MEP, Perrett D (1998) Effects of buffer depletion in capillary electrophoresis: development of a continuous flow cathode. Chromatographia 48(5–6):355–359
Li XF, Ren H, Le X, Qi M, Ireland ID, Dovichi NJ (2000) Migration time correction for the analysis of derivatized amino acids and oligosaccharides by micellar capillary electrochromatography. J Chromatogr A 869(1–2):375–384
Wendeler M, Sandhoff K (2009) Hexosaminidase assays. Glycoconj J 26(8):945–952
Lal A, Haynes SR, Gorospe M (2005) Clean Western blot signals from immunoprecipitated samples. Mol Cell Probes 19(6):385–388
Reddy JK, Hashimoto T (2001) Peroxisomal beta-oxidation and peroxisome proliferator-activated receptor alpha: an adaptive metabolic system. Annu Rev Nutr 21:193–230
Zhou M, Diwu Z, Panchuk-Voloshina N, Haugland RP (1997) A stable nonfluorescent derivative of resorufin for the fluorometric determination of trace hydrogen peroxide: applications in detecting the activity of phagocyte NADPH oxidase and other oxidases. Anal Biochem 253(2):162–168
Kasurinen J (1992) A novel fluorescent fatty acid, 5-methyl-BDY-3-dodecanoic acid, is a potential probe in lipid transport studies by incorporating selectively to lipid classes of BHK cells. Biochem Biophys Res Commun 187(3):1594–1601
Naylor BL, Picardo M, Homan R, Pownall HJ (1991) Effects of fluorophore structure and hydrophobicity on the uptake and metabolism of fluorescent lipid analogs. Chem Phys Lipids 58(1–2):111–119
Brando T, Pardin C, Prandi J, Puzo G (2002) Analysis of aminofluorescein-fatty acid derivatives by capillary electrophoresis with laser-induced fluorescence detection at the attomole level: application to mycobacterial fatty acids. J Chromatogr A 973(1–2):203–210
Sauro VS, Strickland KP (1987) Changes in oleic acid oxidation and incorporation into lipids of differentiating L6 myoblasts cultured in normal or fatty acid-supplemented growth medium. Biochem J 244(3):743–748
Thumser AE, Storch J (2007) Characterization of a BODIPY-labeled fluorescent fatty acid analogue. Binding to fatty acid-binding proteins, intracellular localization, and metabolism. Mol Cell Biochem 299(1–2):67–73
Katzenmeyer JB, Eddy CV, Arriaga EA (2010) Tandem laser-induced fluorescence and mass spectrometry detection for high-performance liquid chromatography analysis of the in vitro metabolism of doxorubicin. Anal Chem 82(19):8113–8120
Chu XP, Zhao T, Zhang YY, Zhao AH, Zhou MM, Zheng XJ, Dan M, Jia W (2009) Determination of 13 free fatty acids in pheretima using ultra-performance LC-ESI-MS. Chromatographia 69(7–8):645–652
Haynes CA, Allegood JC, Sims K, Wang EW, Sullards MC, Merrill AH Jr (2008) Quantitation of fatty acyl-coenzyme As in mammalian cells by liquid chromatography-electrospray ionization tandem mass spectrometry. J Lipid Res 49(5):1113–1125
Magnes C, Sinner FM, Regittnig W, Pieber TR (2005) LC/MS/MS method for quantitative determination of long-chain fatty acyl-CoAs. Anal Chem 77(9):2889–2894
Yamada J, Ogawa S, Horie S, Watanabe T, Suga T (1987) Participation of peroxisomes in the metabolism of xenobiotic acyl compounds: comparison between peroxisomal and mitochondrial beta-oxidation of omega-phenyl fatty acids in rat liver. Biochim Biophys Acta 921(2):292–301
Jakobs BS, Wanders RJ (1995) Fatty acid beta-oxidation in peroxisomes and mitochondria: the first, unequivocal evidence for the involvement of carnitine in shuttling propionyl-CoA from peroxisomes to mitochondria. Biochem Biophys Res Commun 213(3):1035–1041
Acknowledgments
YW acknowledges support through a 2008–2009 Merck Research Laboratories Fellowship in Analytical/Physical Chemistry. THT acknowledges support from NIH grant T32GM008700. EAA acknowledges support from NIH grant AG020866.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in the 10th Anniversary Issue.
Electronic supplementary materials
Below is the link to the electronic supplementary material.
ESM 1
(PDF 890 kb)
Rights and permissions
About this article
Cite this article
Wang, Y., Taylor, T.H. & Arriaga, E.A. Analysis of the bioactivity of magnetically immunoisolated peroxisomes. Anal Bioanal Chem 402, 41–49 (2012). https://doi.org/10.1007/s00216-011-5476-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-011-5476-3