Abstract
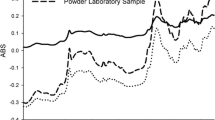
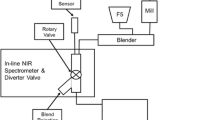
Near-infrared spectroscopy (NIRS) has been widely used in the pharmaceutical field because of its ability to provide quality information about drugs in near-real time. In practice, however, the NIRS technique requires construction of multivariate models in order to correct collinearity and the typically poor selectivity of NIR spectra. In this work, a new methodology for constructing simple NIR calibration models has been developed, based on the spectrum for the target analyte (usually the active principle ingredient, API), which is compared with that of the sample in order to calculate a correlation coefficient. To this end, calibration samples are prepared spanning an adequate concentration range for the API and their spectra are recorded. The model thus obtained by relating the correlation coefficient to the sample concentration is subjected to least-squares regression. The API concentration in validation samples is predicted by interpolating their correlation coefficients in the straight calibration line previously obtained. The proposed method affords quantitation of API in pharmaceuticals undergoing physical changes during their production process (e.g. granulates, and coated and non-coated tablets). The results obtained with the proposed methodology, based on correlation coefficients, were compared with the predictions of PLS1 calibration models, with which a different model is required for each type of sample. Error values lower than 1–2% were obtained in the analysis of three types of sample using the same model; these errors are similar to those obtained by applying three PLS models for granules, and non-coated and coated samples. Based on the outcome, our methodology is a straightforward choice for constructing calibration models affording expeditious prediction of new samples with varying physical properties. This makes it an effective alternative to multivariate calibration, which requires use of a different model for each type of sample, depending on its physical presentation.


Similar content being viewed by others
References
Lafargue ME, Feinberg MH, Daudin JJ, Rutledge DN (2003) Anal Bioanal Chem 375:496–504
Macho S, Larrechi MS (2002) Trends Anal Chem 21:799–806
Jiang B, Dong Huang Y (2008) J Reinf Plast Comp 26:1625–1636
Blanco M, Bautista M, Alcala M (2008) J Pharm Sci 97:1236–1245
Blanco M, Vilarroya I (2002) Trends Anal Chem 21:240–250
Martens H, Naes T (1989) Multivariate calibration. Wiley, London
Frasson Scafi SH, Pasquini C (2001) Analyst 126:2218–2224
Jovanovic N, Gerich A, Bouchard A, Jiskoot W (2006) Pharm Res 23:2002–2013
Moes JJ, Ruijken MM, Gout E, Frijlink HW, Ugwoke MI (2008) Int J Pharm 357:108–118
Patel AD, Luner PE, Kemper MS (2001) J Pharm Sci 90:360–370
Peng Y, Wu P (2004) Polymer 45:5295–5299
Asuero AG, Sayago A, Gonzalez AG (2006) Crit Rev Anal Chem 36:41–59
Blanco M, Bautista M, Alcalà M (2008) AAPS Pharm Sci Technol (in press)
Acknowledgements
The authors are grateful to Spain’s MCyT for funding this research within the framework of Project CTQ2006–12923.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Blanco, M., Cruz, J. & Bautista, M. Development of a univariate calibration model for pharmaceutical analysis based on NIR spectra. Anal Bioanal Chem 392, 1367–1372 (2008). https://doi.org/10.1007/s00216-008-2426-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-008-2426-9