Abstract
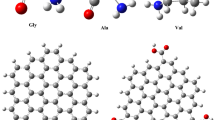
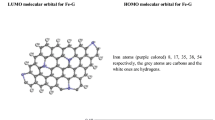
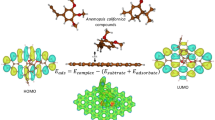
Density functional theory calculations are carried out to understand the interactions of selected amino acids with various nitrogen-containing graphene sheets. Three types of N-containing graphene, namely pyridinic N, pyrrolic N, and graphitic N, are considered. As compared to the pristine graphene, it is found that the graphitic N could slightly enhance the interaction between amino acid and the substrate. The pyridinic N exhibits the best affinity toward the amino acids, and the increase in nitrogen content could result in more polarization in the substrate and effectively improve the interaction. In addition, the interactions between the amino acids and the hydrogenated pyridinic N and pyrrolic N in the plane and on the edge of graphene are also studied. The interaction is weakened by the hydrogenated N atom in the plane while it remains strongly for the hydrogenated N atom on the edge. The calculated charge distribution of the various nitrogen-containing graphene sheets has been illustrated, and the result shows that the pyridinic N in the plane and the hydrogenated N atom on the edge have better affinity toward the studied amino acids. This study would be useful for the practical applications of biosensor and drug delivery.









Similar content being viewed by others
References
Allen MJ, Tung VC, Kaner RB (2010) Honeycomb carbon: a review of graphene. Chem Rev 110:132–145
Balandin AA, Ghosh S, Bao WZ, Calizo I, Teweldebrhan D, Miao F, Lau CN (2008) Superior thermal conductivity of single-layer graphene. Nano Lett 8:902–907
Bunch JS, van der Zande AM, Verbridge SS, Frank IW, Tanenbaum DM, Parpia JM, Craighead HG, McEuen PL (2007) Electromechanical resonators from graphene sheets. Science 315:490–493
Zhang YB, Tan YW, Stormer HL, Kim P (2005) Experimental observation of the quantum Hall effect and Berry’s phase in graphene. Nature 438:201–204
Zhu YW, Murali S, Stoller MD, Ganesh KJ, Cai WW, Ferreira PJ, Pirkle A, Wallace RM, Cychosz KA, Thommes M, Su D, Stach EA, Ruoff RS (2011) Carbon-based supercapacitors produced by activation of graphene. Science 332:1537–1541
Feng LZ, Liu ZA (2011) Graphene in biomedicine: opportunities and challenges. Nanomedicine 6:317–324
Kuila T, Bose S, Khanra P, Mishra AK, Kim NH, Lee JH (2011) Recent advances in graphene-based biosensors. Biosens Bioelectron 26:4637–4648
Elias DC, Nair RR, Mohiuddin TMG, Morozov SV, Blake P, Halsall MP, Ferrari AC, Boukhvalov DW, Katsnelson MI, Geim AK, Novoselov KS (2009) Control of graphene’s properties by reversible hydrogenation: evidence for graphane. Science 323:610–613
Novoselov KS, Geim AK, Morozov SV, Jiang D, Katsnelson MI, Grigorieva IV, Dubonos SV, Firsov AA (2005) Two-dimensional gas of massless dirac fermions in graphene. Nature 438:197–200
Gagner JE, Shrivastava S, Qian X, Dordick JS, Siegel RW (2012) Engineering nanomaterials for biomedical applications requires understanding the nano-bio interface: a perspective. J Phys Chem Lett 3:3149–3158
Gao J, Xu B (2009) Applications of nanomaterials inside cells. Nano Today 4:37–51
Vashist SK, Luong JHT (2015) Recent advances in electrochemical biosensing schemes using graphene and graphene-based nanocomposites. Carbon 84:519–550
Duan J, Chen S, Jaroniec M, Qiao SZ (2015) Heteroatom-doped graphene-based materials for energy-relevant electrocatalytic processes. ACS Catal 5:5207–5234
Fan X, Zhang G, Zhang F (2015) Multiple roles of graphene in heterogeneous catalysis. Chem Soc Rev 44:3023–3035
Hao G-P, Li W-C, Qian D, Lu A-H (2010) Rapid synthesis of nitrogen-doped porous carbon monolith for CO2 capture. Adv Mater 22:853–857
Park Y, Yoo J, Lim B, Kwon W, Rhee S-W (2016) Improving the functionality of carbon nanodots: doping and surface functionalization. J Mater Chem A 4:11582–11603
Shih Y-H, Chen J-H, Lin Y, Chen H-T, Lin C-H, Huang H-Y (2017) Nitrogen-doped porous carbon material derived from metal-organic gel for small biomolecular sensing. Chem Commun 53:5725–5728
Tiwari JN, Vij V, Kemp KC, Kim KS (2016) Engineered carbon-nanomaterial-based electrochemical sensors for biomolecules. ACS Nano 10:46–80
Ayala P, Arenal R, Rümmeli M, Rubio A, Pichler T (2010) The doping of carbon nanotubes with nitrogen and their potential applications. Carbon 48:575–586
Majeed S, Zhao J, Zhang L, Anjum S, Liu Z, Xu G (2013) Synthesis and electrochemical applications of nitrogen-doped carbon nanomaterials. Nanotechnol Rev 2:615–635
Gong K, Du F, Xia Z, Durstock M, Dai L (2009) Nitrogen-doped carbon nanotube arrays with high electrocatalytic activity for oxygen reduction. Science 323:760–764
Matanovic I, Artyushkova K, Strand MB, Dzara MJ, Pylypenko S, Atanassov P (2016) Core level shifts of hydrogenated pyridinic and pyrrolic nitrogen in the nitrogen-containing graphene-based electrocatalysts: in-plane vs edge defects. J Phys Chem C 120:29225–29232
Shari T, Hu G, Jia X, Wagberg T (2012) Formation of active sites for oxygen reduction reactions by transformation of nitrogen functionalities in nitrogen-doped carbon nanotubes. ACS Nano 6:8904–8912
Singla P, Riyaz M, Singhal S, Goel N (2016) Theoretical study of adsorption of amino acids on graphene and BN sheet in gas and aqueous phase with empirical DFT dispersion correction. Phys Chem Chem Phys 18:5597–5604
Vovusha H, Sanyal S, Sanyal B (2013) Interaction of nucleobases and aromatic amino acids with graphene oxide and graphene flakes. J Phys Chem Lett 4:3710–3718
Mukhopadhyay S, Scheicher RH, Pandey R, Karna SP (2011) Sensitivity of boron nitride nanotubes toward biomolecules of different polarities. J Phys Chem Lett 2:2442–2447
Mallakpour S, Abdolmale A, Borandeh S (2014) Covalently functionalized graphene sheets with biocompatible natural amino acids. Appl Surf Sci 307:533–542
Farmanzadeh D, Ghazanfary S (2014) BNNTs under the influence of external electric field as potential new drug delivery vehicle of Glu, Lys, Gly and Ser amino acids: a first-principles study. Appl Surf Sci 320:391–399
Wang Q, Arash B (2014) A review on applications of carbon nanotubes and graphenes as nano-resonator sensors. Comput Mater Sci 82:350–360
Mukhopadhyay S, Gowtham S, Scheicher RH, Pandey R, Karna SP (2010) Theoretical study of physisorption of nucleobases on boron nitride nanotubes: a new class of hybrid nano-biomaterials. Nanotechnology 21:165703–165709
Farmanzadeh D, Ghazanfary S (2014) Interaction of vitamins A, B1, C, B3 and D with zigzag and armchair boron nitride nanotubes: a DFT study. C R Chim 17:985–993
Anota EC, Cocoletzi GH (2014) Low-dimensional systems and nanostructures. Phys E 56:134–140
Nam SW, Jung C, Li H, Yu M, Flora JRV, Boateng LK, Her N, Zoh KD, Yoon Y (2015) Adsorption characteristics of diclofenac and sulfamethoxazole to graphene oxide in aqueous solution. Chemosphere 136:20–26
Martinek K, Mozhaev VV (1985) Immobilization of enzymes: an approach to fundamental studies in biochemistry. Adv Enzymol Relat Areas Mol Biol 57:179–249
Vandamme EJ (1983) Peptide antibiotic production through immobilized biocatalyst technology. Enzyme Microb Technol 5:403–415
Rimola A (2015) Intrinsic ladders of affinity for amino-acid-analogues on boron nitride nanomaterials: a B3LYP-D2* periodic study. J Phys Chem C 119:17707–17717
Perdew JP, Burke K, Ernzerhof M (1996) Generalized gradient approximation made simple. Phys Rev Lett 77:3865–3868
Perdew JP, Wang Y (1992) Accurate and simple analytic representations of the electron–gas correlation energy. Phys Rev B Condens Matter Mater Phys 45:13244–13249
Delley B (2000) From molecules to solids with the Dmol3 approach. J Chem Phys 113:7756
Grimme S (2004) Accurate description of van der Waals complexes by density functional theory including empirical corrections. J Comput Chem 25:1463–1473
Grimme S (2006) Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J Comput Chem 27:1787–1799
Lazar P, Zboril R, Pumera M, Otyepka M (2014) Chemical nature of boron and nitrogen dopant atoms in graphene strongly influences its electronic properties. Phys Chem Chem Phys 16:14231–14235
Acknowledgements
H.-T. C. thanks the Ministry of Science and Technology (MOST) under Grant Numbers MOST 107-2113-M-033-004, 106-2113-M-033-003, MOST 105-2113-M-033-008, and MOST 104-2113-M-033-010, Chung Yuan Christian University (CYCU), and National Center for Theoretical Sciences (NCTS), Taiwan, for supporting this work and the use of facilities at the National Center for High-Performance Computing, Taiwan.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chen, JH., Chen, HT. Computational explanation for interaction between amino acid and nitrogen-containing graphene. Theor Chem Acc 137, 176 (2018). https://doi.org/10.1007/s00214-018-2392-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00214-018-2392-z