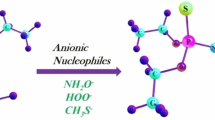
Abstract
Density functional theory (DFT) has been used to study the solvolysis process of the organophosphorus compound P-[2-(dimethylamino)ethyl]-N,N-dimethylphosphonamidic fluoride (GV) with simple nucleophile [hydroxide (HO−)] and α-nucleophiles [hydroperoxide (HOO−) and hydroxylamine anion (NH2O−)]. The lowest energy conformer of GV used for the solvolysis process was identified with Monte Carlo conformational search (MCMM) algorithm employing MMFFs force field followed by DFT calculations. The profound effect was found for α-nucleophiles toward the solvolysis of GV compared to normal alkaline hydrolysis. Incorporation of solvent (water) employing SCRF (PCM) model at B3LYP/6-31+G* showed that solvolysis of GV with hydroperoxide (activation energy = 7.6 kcal/mol) is kinetically more favored compared to hydroxide and hydroxylamine anion (activation energy = 11.0 and 9.2 kcal/mol, respectively). The faster solvolysis of GV with hydroperoxide is achieved due to strong intermolecular hydrogen bonding in the transition state geometry compared to similar α-nucleophile hydroxylamine anion. Assistance of a water molecule in solvolysis of GV affects the activation barriers; however, the hydroperoxidolysis remains the preferential process. The topological properties of electron density distributions for (–X–H···O, X = O, N) intermolecular hydrogen bonding bridges have been analyzed in terms of Bader theory of atoms in molecules (AIM). Further, the analysis was extended by natural bond orbital (NBO) methods for the strength of intermolecular hydrogen bonding in the transition state geometries. This study showed that the reactivity of these α-nucleophiles toward the solvolysis of GV is a delicate balance between the nucleophilicity and hydrogen-bond strength. Solvation governs the overall thermodynamics for the destruction of GV, which otherwise is unfavored in the gas phase studies.









Similar content being viewed by others
References
Bunton CA (1997) Chemical warfare. In: Lagowsky JJ (ed) Macmillan encyclopedia of chemistry. Macmillan Reference USA, vol 1. Simon and Schuster Macmillan, New York, pp 343–346
DeFrank JJ (1991) Organophosphorus cholinesterase inhibitors: detoxification by microbial enzymes. In: Kelly JW, Baldwin TO (eds) Applications of enzyme biotechnolgy. Plenum Press, New York, pp 165–180
Heilbronn-Wikstrom E (1965) Sven Kem Tidskr 77:598–631
Kolb HC, Sharpless KB (2003) Drug Discov Today 8:1128–1137
Quinn DM (1987) Chem Rev 87:955–979
Shafferman A, Kronman C, Flashner Y, Leitner M, Grosfeld H, Ordentlich A, Gozes Y, Cohen S, Ariel N, Barak D, Harel M, Silman I, Sussman JL, Velan B (1992) J Biol Chem 267:17640–17648
Wang J, Roszak S, Gu J, Leszczynski J (2005) J Phys Chem B 109:1006–1014
Wang J, Gu J, Leszczynski J (2006) J Phys Chem B 110:7567–7573
Taylor P, Lappi S (1975) Biochemistry 14:1989–1997
Kumar VP, Ganguly B, Bhattacharya S (2004) J Org Chem 69:8634–8642
Gershonov E, Columbus I, Zafrani Y (2009) J Org Chem 74:329–338
Yang YC, Szafraniec LL, Beaudry WT, Rohrbaugh DK (1990) J Am Chem Soc 112:6621–6627
Simanenko YS, Savelova VA, Prokop’eva TM, Mikhailov VA, Turovskaya MK, Karpichev EA, Popov AF, Gillitt ND, Bunton CA (2004) J Org Chem 69:9238–9240
Vorontsov AV, Davydov L, Reddy EP, Lion C, Savinov EN, Smirniotis PG (2002) New J Chem 26:732–744
Vorontsov AV, Chen YC, Smirniotis PG (2004) J Hazard Mat B113:89–95
Michalkova A, Gorb L, IIchenko GM, Zhikol OA, Shishkin OV, Leszczynski J (2004) J Phys Chem B 108:1918–1930
Keizer TS, Pue De LJ, Parkin S, Atwood DA (2002) J Am Chem Soc 124:1864–1865
Hill CM, Li WS, Thoden JB, Holden HM, Raushel FM (2003) J Am Chem Soc 125:8990–8991
Amitai G, Adani R, Hershkovitz M, Bel P, Rabinovitz I, Meshulam H (2003) J Appl Toxicol 23:225–233
Hoskins FCG, Walker JE, Dettbarn WD, Wild JR (1995) Biochem Pharmacol 49:711–715
Kiddle JJ, Mezyk SP (2004) J Phys Chem B 108:9568–9570
Aguila A, O’Shea KE, Tobien T, Asmus KD (2001) J Phys Chem A 105:7834–7839
Hoenig SL (2007) Compendium of chemical warfare agents. Springer, New York, p 100
Royo S, Martínez-Máñez R, Sancenón F, Costero AM, Parra M, Gil S (2007) Chem Commun 4839–4847
Cassagne T, Cristau HJ, Delmas G, Desgranges M, Lion C, Magnaud G, Torreilles É, Virieux D (2001) Heteroat Chem 12:485–490
Bermudez VM (2007) J Phys Chem C 111:9314–9323
Bandyopadhyay I, Kim MJ, Lee YS, Churchill DG (2006) J Phys Chem A 110:3655–3661
Šečkutė J, Menke JL, Emnett RJ, Patterson EV, Cramer CJ (2005) J Org Chem 70:8649–8660
Zheng F, Zhan CG, Ornstein RL (2001) J Chem Soc Perkin Trans 2:2355–2363
Patterson EV, Cramer CJ (1998) J Phys Org Chem 11:232–240
Daniel KA, Kopff LA, Patterson EV (2008) J Phys Org Chem 21:321–328
Menke JL, Patterson EV (2007) J Mol Struct: THEOCHEM 811:281–291
Khan MAS, Kesharwani MK, Bandyopadhyay T, Ganguly B (2009) J Mol Graphics Modell 28:177–182
Kassa J, Bajgar J (1996) Acta Med 39:27–30
Chang G, Guida WC, Still WC (1989) J Am Chem Soc 111:4379–4386
Saunders M, Houk KN, Wu YD, Still WC, Lipton M, Chang G, Guida WC (1990) J Am Chem Soc 112:1419–1427
Halgren TA (1996) J Comput Chem 17:616–641
Halgren TA (1996) J Comput Chem 17:553–586
Halgren TA (1996) J Comput Chem 17:520–552
Halgren TA (1996) J Comput Chem 17:490–519
Halgren TA, Nachbar RB (1996) J Comput Chem 17:587–615
Mohamdi F, Richards NGJ, Guida WC, Liskamp R, Lipton M, Caufield C, Chang G, Hendrickson T, Still WC (1990) J Comput Chem 11:440–467
Polak E, Ribiere G (1969) Rev Fr Inf Rech Oper 16-R1:35–43
Shenkin PS, McDonald DQ (1994) J Comput Chem 15:899–916
Hillson SD, Smith E, Zeldin M, Parish CA (2005) J Phys Chem A 109:8371–8378
Becke AD (1993) J Chem Phys 98:5648–5652
Lee C, Yang W, Parr RG (1988) Phys Rev B 37:785–789
Beck JM, Hadad CM (2008) Chemico biological interactions 175:200–203
Hehre WJ, Radom L, Schleyer PvR, Pople JA (1988) Ab initio molecular orbital theory. Wiley, New York
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Montgomery JA Jr, Vreven T, Kudin KN, Burant JC, Millam JM, Iyengar SS, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson GA, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox JE, Hratchian HP, Cross JB, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Ayala PY, Morokuma K, Voth GA, Salvador P, Dannenberg JJ, Zakrzewski VG, Dapprich S, Daniels AD, Strain MC, Farkas O, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Ortiz JV, Cui Q, Baboul AG, Clifford S, Cioslowski J, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Gonzalez C, Pople JA (2004) Gaussian 03, Revision E.01. Gaussian, Inc., Wallingford, CT
Tomasi J, Persico M (1994) Chem Rev 94:2027–2094
Cossi M, Barone V, Cammi R, Tomasi J (1996) Chem Phys Lett 255:327–335
Barone V, Cossi M, Tomasi J (1997) J Chem Phys 107:3210–3221
Barone V, Cossi M, Tomasi J (1998) J Comput Chem 19:404–417
Cossi M, Barone V (1998) J Chem Phys 109:6246–6254
Zhao Y, Lynch BJ, Truhlar DG (2004) J Phys Chem A 108:2715–2719
Zhao Y, Lynch BJ, Truhlar DG (2005) Phys Chem Chem Phys 7:43–52
Zhao Y, Schultz NE, Truhlar DG (2006) J Chem Theory Comput 2:364–382
Larsson L (1957) Acta Chem Scand 11:1131–1142
González C, Schlegel HB (1990) J Phys Chem 94:5523–5527
González C, Schlegel HB (1991) J Chem Phys 95:5853–5860
Bader RFW (1990) Atoms in molecule: a quantum theory. Oxford University Press, New York
Cioslowski J, Nanayakkara A, Challacombe M (1993) Chem Phys Lett 203:137–142
Cioslowski J (1994) Chem Phys Lett 219:151–154
Espinosa E, Souhassou M, Lachekar H, Lecomte C (1999) Acta Crystallogr B 55:563–572
Glendening DE, Reed AE, Carpenter JE, Weinhold F (1992) NBO Version 3.1
Thatcher GRJ, Kluger R (1989) Adv Phys Org Chem 25:99
Zhan C-G, Landry DW, Ornstein RL (2000) J Am Chem Soc 122:1522–1530
van Bochove MA, Bickelhaupt FM (2008) Eur J Org Chem 649–654
van Bochove MA, Swart M, Bickelhaupt FM (2007) Chem Phys Chem 8:2452–2463
van Bochove MA, Swart M, Bickelhaupt FM (2006) J Am Chem Soc 128:10738–10744
Kirby AJ, Manfredi AM, Souza BS, Medeiros M, Priebe JP, Brandão TAS, Nome F (2009) ARKIVOC. (iii):28–38
Kirby AJ, Souza BS, Medeiros M, Priebe JP, Manfredi AM, Nome F (2008) Chem Commun 4428-4429
Kirby AJ, Davies JE, Brandão TAS, da Silva PF, Rocha WR, Nome F (2006) J Am Chem Soc 128:12374–12375
Anslyn EV, Dougherty DA (2006) Modern physical organic chemistry. University Science Books, Sausalito, p 168
Raissi H, Jalbout AF, Farsi H, Abbasi B, De Leon A, Moghiminia S (2009) Int J Quantum Chem 109:1609–1616
Wiberg KB (1968) Tetrahedron 24:1083–1096
Popelier PLA, Bader RFW (1992) Chem Phys Lett 189:542–548
Nowroozi A, Jalbout AF, Roohi H, Khalilinia E, Sadeghi M, De Leon A, Raissi H (2009) Int J Quantum Chem 109:1505–1514
Howard ST (2000) J Am Chem Soc 122:8238–8244
Simanenko YS, Popov AF, Prokop’eva TM, Savelova VA, Belousova IA (1994) Theor Exp Chem 30:61–64
Acknowledgments
Authors thank DAE-BRNS, Mumbai, India for financial support of this work. One of the authors M.K.K. is thankful to UGC, New Delhi, India for awarding fellowship.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kesharwani, M.K., Khan, M.A.S., Bandyopadhyay, T. et al. Solvolysis process of organophosphorus compound P-[2-(dimethylamino)ethyl]-N,N-dimethylphosphonamidic fluoride with simple and α-nucleophiles: a DFT study. Theor Chem Acc 127, 39–47 (2010). https://doi.org/10.1007/s00214-009-0701-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00214-009-0701-2