Abstract
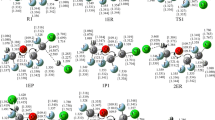
The multiple-channel reactions Cl + Si(CH3)4 and Br + Si(CH3)4 are investigated by direct dynamics method. The minimum energy path is calculated at the MP2/6-31+G(d,p) level, and energetic information is further refined by the MC-QCISD (single-point) method. The rate constants for individual reaction channel are calculated by the improved canonical variational transition state theory with small-curvature tunneling correction over the temperature range 200–3,000 K. The theoretical three-parameter expression k 1(T) = 9.97 × 10−13 T 0.54exp(613.22/T) and k 2(T) = 1.16 × 10−17 T 2.30exp(−3525.88/T) (in unit of cm3 molecule−1 s−1) are given. Our calculations indicate that hydrogen abstraction channel is the major channel due to the smaller barrier height among feasible channels considered.




Similar content being viewed by others
References
Calvert JG, Atkinson R, Kerr JA, Madronich S, Moortgat GK, Wallington TJ, Yarwood G (2000) The mechanisms of atmospheric oxidation of the alkenes. Oxford, New York
Calvert JG, Atkinson R, Becker KH, Kamens RH, Seinfeld JH, Wallington TJ, Yarwood G (2002) The mechanisms of atmospheric oxidation of the aromatic hydrocarbons. Oxford, New York
Zhang H, Zhang GL, Wang Y, Yu XY, Liu B, Liu JY, Li ZS (2008) Theo Chem Acc 119:319
Goumri A, Yuan J, Hommel EL, Marshall P (2003) Chem Phys Lett 375:149
Atkinson R (1991) Environ Sci Technol 25:863
Sommerlade R, Parlar H, Wrobel D, Kochs P (1993) Environ Sci Technol 27:2435
Tuazon EC, Aschmann SM, Atkinson R (2000) Environ Sci Technol 34:1970
Bell RL, Truong TN (1994) J Chem Phys 101:10442
Truong TN, Duncan WT, Bell RL (1996) Chemical applications of density functional theory. American Chemical Society, Washington, DC, p 85
Truhlar DG (1995) In: Heidrich D (ed) The reaction path in chemistry: current approaches and perspectives. Kluwer, Dordrecht, The Netherlands, p 229
Corchado JC, Espinosa-Garcia J, Hu W-P, Rossi I, Truhlar DG (1995) J Phys Chem 99:687
Hu W-P, Truhlar DG (1996) J Am Chem Soc 118:860
Fast PL, Truhlar DG (2000) J Phys Chem A 104:6111
Corchado JC, Chuang Y-Y, Fast PL, Villa J, Hu W-P, Liu Y-P, Lynch GC, Nguyen KA, Jackels CF, Melissas VS, Lynch BJ, Rossi I, Coitino EL, Ramos AF, Pu J, Albu TV (2002) POLYRATE version 9.1. Department of Chemistry and Supercomputer Institute, University of Minnesota, Minneapolis, Minnesota
Truhlar DG, Garrett BC (1980) Acc Chem Res 13:440
Truhlar DG, Isaacson AD, Garrett BC (1985) Generalized transition state theory. In: Baer M (ed) The theory of chemical reaction dynamics, vol 4. CRC Press, Boca Raton, FL, p 65
Duncan WT, Truong TN (1995) J Chem Phys 103:9642
Frisch MJ, Head-Gordon M, Pople JA (1990) Chem Phys Lett 166:275
Head-Gordon M, Pople JA, Frisch MJ (1988) Chem Phys Lett 153:503
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Montgomery JA Jr, Vreven T, Kudin KN, Burant JC, Millam JM, Iyengar SS, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson GA, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox JE, Hratchian HP, Cross JB, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Ayala PY, Morokuma K, Voth GA, Salvador P, Dannenberg JJ, Zakrzewski VG, Dapprich S, Daniels AD, Strain MC, Farkas O, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Ortiz JV, Cui Q, Baboul AG, Clifford S, Cioslowski J, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Gonzalez C, Pople JA (2003) Gaussian, Inc., Pittsburgh, PA
Garrett BC, Truhlar DG, Grev RS, Magnuson AW (1980) J Phys Chem 84:1730
Lu DH, Truong TN, Melissas VS, Lynch GC, Liu YP, Grarrett BC, Steckler R, Issacson AD, Rai SN, Hancock GC, Lauderdale JG, Joseph T, Truhlar DG (1992) Comput Phys Commun 71:235
Liu Y-P, Lynch GC, Truong TN, Lu D-H, Truhlar DG, Garrett BC (1993) J Am Chem Soc 115:2408
Truhlar DG (1991) J Comput Chem 12:266
Chuang YY, Truhlar DG (2000) J Chem Phys 112:1221
Kuchitsu K (1998) In: Structure of free polyatomic molecules basic data, vol 1. Springer, Berlin, p 104
NIST Chemistry WebBook, NIST Standard Reference Database Number 69, June 2005 Release, Data compiled by K. P. Huber and G. Herzberg
In NIST Chemistry WebBook, NIST Standard Reference Database Number 69, June 2005 Release, Vibrational frequency date compiled by Coblentz Society, Inc
In NIST Chemistry WebBook, NIST Standard Reference Database Number 69, June 2005 Release, Vibrational frequency date compiled by T. Shimanouchi
Chase MW Jr, Davies CA, Downey JR, Fryrip DJ, McDonald RA, Syverud AN (1985) JANAF thermochemical tables. Ref Data 14(Suppl 1):1
Chase MW (1998) NIST-JANAF themochemical tables. J Phys Chem Ref Data Monograph 9:1–1951
Hammond GS (1955) J Am Chem Soc 77:334
Ding L, Marshall P (1992) J Am Chem Soc 114:5754
Ferguson KC, Okafo EN, Whittle E (1973) J Chem Soc Faraday Trans 1(69):295
Zhang H, Zhang GL, Liu JY, Miao S, Liu B, Li ZS (2009) J Comput Chem 30:236
Zhang H, Zhang GL, Liu JY, Liu CY, Liu B, Li ZS (2009) Theor Chem Acc 122:107
Phillips JA, Cramer CJ (2007) J Phys Chem B 111:1408
Zhang H, Zhang GL, Wang Y, Yu XY, Liu B, Liu JY, Li ZS (2008) Theor Chem Acc 119:319
Lynch BJ, Zhao Y, Truhlar DG (2005) J Phys Chem A 109:1643
Walsh R (1992) In: Martinho Simões JA (ed) Energetica of organometallic species; NATO-ASI Series C, 367. Kluwer, Dordrecht (Chap 11)
Acknowledgments
The authors thank Professor Donald G. Truhlar for providing POLYRATE 9.1 program. This work is supported by the National Natural Science Foundation of China (20333050, 20303007, 50743013, and 20973049), the Program for New Century Excellent Talents in University (NCET), the Key subject of Science and Technology by the Ministry of Education of China, the Key subject of Science and Technology by Jilin Province, the Foundation for University Key Teacher by the Department of Education of Heilongjiang Province (1152G010), the SF of Graduate Innovation by Department of Education of Heilongjiang province (YJSCX2009-055HLJ), the SF for Postdoctoral of HLJ province (LBH-Q07058), and Natural Science Foundation of Heilongjiang Province (B200605).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, H., Zhang, Gl., Liu, Jy. et al. Dual-level direct dynamics studies on the reactions of tetramethylsilane with chlorine and bromine atoms. Theor Chem Acc 125, 75–82 (2010). https://doi.org/10.1007/s00214-009-0664-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00214-009-0664-3