Abstract
Rationale
Arginine vasopressin (AVP) is a neuropeptide that modulates both physiological and emotional responses to threat. Until recently, drugs that target vasopressin receptors (V1a) in the human central nervous system were unavailable. The development of a novel V1a receptor antagonist, SRX246, permits the experimental validation of vasopressin’s role in the regulation of anxiety and fear in humans.
Objectives
Here, we examined the effects of SRX246 in a proof-of-concept translational paradigm of fear (phasic response to imminent threat) and anxiety (prolonged response to potential threat).
Methods
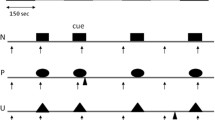
Healthy volunteers received both SRX246 and placebo in a randomized, double-blind, counter-balanced order separated by a 5–7-day wash-out period. Threat consisted of unpleasant electric shocks. The “NPU” threat test probed startle reactivity during predictable threat (i.e., fear-potentiated startle) and unpredictable threat (i.e., anxiety-potentiated startle).
Results
As predicted, SRX246 decreased anxiety-potentiated startle independent of fear-potentiated startle.
Conclusions
As anxiety-potentiated startle is elevated in anxiety and trauma-associated disorders and decreased by traditional anxiolytics such as SSRIs and benzodiazepines, the V1a receptor is a promising novel treatment target.


adapted from Schmitz and Grillon 2012). b Participants were asked to rate their online subjective anxiety on a scale of 1–10 by pressing arrows on the keyboard throughout the NPU threat test. During the N condition, the display read “No Shocks at Anytime” at the top of the screen; during the P condition, the display read “Shocks Only During Cue”; and during the U condition, the display read “Shocks at Anytime.” The visual threat cues consisted of colored shapes that were displayed intermittently throughout each condition. Startle probes are represented by yellow bursts, and shocks are represented by red lightning

Similar content being viewed by others
References
Åkerlund M, Bossmar T, Brouard R et al (1999) Receptor binding of oxytocin and vasopressin antagonists and inhibitory effects on isolated myometrium from preterm and term pregnant women. Br J Ostet Gynaecol 106:1047–1053
Anderzhanova E, Kirmeier T, Wotjak CT (2017) Animal models in psychiatric research: the RDoC system as a new framework for endophenotype-oriented translational neuroscience. Neurobiol Stress 7:47–56
Avery SN, Clauss JA, Blackford JU (2016) The human BNST: functional role in anxiety and addiction. Neuropsychopharmacology 41:126–141
Baas JMP, Grillon C, Böcker KBE et al (2002) Benzodiazepines have no effect on fear-potentiated startle in humans. Psychopharmacology 161:233–247
Ballard ED, Ionescu DF, Vande VJL et al (2014) Increased fear-potentiated startle in major depressive disorder patients with lifetime history of suicide attempt. J Affect Disord 162:34–38
Barlow DH (2000) Unraveling the mysteries of anxiety and its disorders from the perspective of emotion theory. Am Psychol 55:1247–1263
Bayerl DS, Hönig JN, Bosch OJ (2016) Vasopressin V1a, but not V1b, receptors within the PVN of lactating rats mediate maternal care and anxiety-related behaviour. Behav Brain Res 305:18–22
Berger W, Mendlowicz MV, Marques-Portella C et al (2009) Pharmacologic alternatives to antidepressants in posttraumatic stress disorder: a systematic review. Prog Neuropsychopharmacol Biol Psychiatry 33:169–180
Bielsky IF, Hu S-B, Szegda KL et al (2004) Profound impairment in social recognition and reduction in anxiety-like behavior in vasopressin V1a receptor knockout mice. Neuropsychopharmacology 29:483–493
Bleickardt CJ, Mullins DE, MacSweeney CP et al (2009) Characterization of the V1a antagonist, JNJ-17308616, in rodent models of anxiety-like behavior. Psychopharmacology 202:711–718
Brinkmann L, Buff C, Feldker K et al (2017a) Distinct phasic and sustained brain responses and connectivity of amygdala and bed nucleus of the stria terminalis during threat anticipation in panic disorder. Psychol Med 47:2675–2688
Brinkmann L, Buff C, Neumeister P et al (2017b) Dissociation between amygdala and bed nucleus of the stria terminalis during threat anticipation in female post-traumatic stress disorder patients. Hum Brain Mapp 38:2190–2205
Brunnlieb C, Münte TF, Tempelmann C, Heldmann M (2013) Vasopressin modulates neural responses related to emotional stimuli in the right amygdala. Brain Res 1499:29–42
Caldwell HK (2017) Oxytocin and vasopressin: powerful regulators of social behavior. Neurosci 23:517–528
Caldwell HK, Lee HJ, Macbeth AH, Young WS III (2008) Vasopressin: behavioral roles of an “original” neuropeptide. Prog Neurobiol 84:1–24
Carter CS (2017) The oxytocin-vasopressin pathway in the context of love and fear. Front Endocrinol (Lausanne) 8:1–12
Colloca L, Pine DS, Ernst M et al (2016) Vasopressin boosts placebo analgesic effects in women: a randomized trial. Biol Psychiatry 79:794–802
Davis M, Walker DL, Miles L, Grillon C (2010) Phasic vs sustained fear in rats and humans: role of the extended amygdala in fear vs anxiety. Neuropsychopharmacology 35:105–135
De Bellis MD, Gold PW, Geracioti TD Jr et al (1993) Association of fluoxetine treatment with reductions in CSF concentrations of corticotropin-releasing hormone and arginine vasopressin in patients with major depression. Am J Psychiatry 150:656–657
de Kloet C, Vermetten E, Geuze E et al (2008) Elevated plasma arginine vasopressin levels in veterans with posttraumatic stress disorder. J Psychiatr Res 42:192–198
Dumais KM, Veenema AH (2016) Vasopressin and oxytocin receptor systems in the brain: sex differences and sex-specific regulation of social behavior. Front Neuroendocrinol 40:1–23
Egashira N, Tanoue A, Matsuda T et al (2007) Impaired social interaction and reduced anxiety-related behavior in vasopressin V1a receptor knockout mice. Behav Brain Res 178:123–127
Feldman R, Vengrober A, Ebstein R (2014) Affiliation buffers stress: cumulative genetic risk in oxytocin-vasopressin genes combines with early caregiving to predict PTSD in war-exposed young children. Transl Psychiatry 4:e370
First MB (2002) The DSM series and experience with DSM-IV. Psychopathology 35:67–71
Godino A, Renard GMM (2018) Effects of alcohol and psychostimulants on the vasopressin system: behavioural implications. J Neuroendocrinol 30:e12611
Gorka SM, Nelson BD, Sarapas C et al (2013a) Relation between respiratory sinus arrythymia and startle response during predictable and unpredictable threat. J Psychophysiol 27:95–105
Gorka SM, Nelson BD, Shankman SA (2013b) Startle response to unpredictable threat in comorbid panic disorder and alcohol dependence. Drug Alcohol Depend 132:216–222
Gorka SM, Liu H, Sarapas C, Shankman SA (2015) Time course of threat responding in panic disorder and depression. Int J Psychophysiol 98:87–94
Gorka SM, Lieberman L, Klumpp H et al (2017a) Reactivity to unpredictable threat as a treatment target for fear-based anxiety disorders. Psychol Med 47:2450–2460
Gorka SM, Lieberman L, Shankman SA, Phan KL (2017b) Startle potentiation to uncertain threat as a psychophysiological indicator of fear-based psychopathology: an examination across multiple internalizing disorders. J Abnorm Psychol 126:8–18
Griebel G, Holmes A (2013) 50 years of hurdles and hope in anxiolytic drug discovery. Nat Rev Drug Discov 12:667–687
Grillon C (2002) Associative learning deficits increase symptoms of anxiety in humans. Biol Psychiatry 51:851–858
Grillon C, Baas J (2003) A review of the modulation of the startle reflex by affective states and its application in psychiatry. Clin Neurophysiol 114:1557–1579
Grillon C, Ameli R, Goddard A et al (1994) Baseline and fear-potentiated startle in panic disorder patients. Biol Psychiatry 35:431–439
Grillon C, Morgan CA III, Davis M, Southwick SM (1998) Effects of experimental context and explicit threat cues on acoustic startle in Vietnam veterans with posttraumatic stress disorder. Biol Psychiatry 44:1027–1036
Grillon C, Baas JMP, Pine DS et al (2006) The benzodiazepine alprazolam dissociates contextual fear from cued fear in humans as assessed by fear-potentiated startle. Biol Psychiatry 60:760–766
Grillon C, Levenson J, Pine DS (2007) A single dose of the selective serotonin reuptake inhibitor citalopram exacerbates anxiety in humans: a fear-potentiated startle study. Neuropsychopharmacology 32:225–231
Grillon C, Lissek S, Rabin S et al (2008) Increased anxiety during anticipation of unpredictable but not predictable aversive stimuli as a psychophysiologic marker of panic disorder. Am J Psychiatry 165:898–904
Grillon C, Chavis C, Covington MF, Pine DS (2009a) Two-week treatment with the selective serotonin reuptake inhibitor citalopram reduces contextual anxiety but not cued fear in healthy volunteers: a fear-potentiated startle study. Neuropsychopharmacology 34:964–971
Grillon C, Pine DS, Lissek S et al (2009b) Increased anxiety during anticipation of unpredictable aversive stimuli in posttraumatic stress disorder but not in generalized anxiety disorder. Biol Psychiatry 66:47–53
Grillon C, Heller R, Hirschhorn E et al (2011) Acute hydrocortisone treatment increases anxiety but not fear in healthy volunteers: a fear-potentiated startle study. Biol Psychiatry 69:549–555
Grillon C, Hale E, Lieberman L et al (2015) The CRH1 antagonist GSK561679 increases human fear but not anxiety as assessed by startle. Neuropsychopharmacology 40:1064–1071
Grillon C, Robinson OJ, Cornwell B, Ernst M (2019) Modeling anxiety in healthy humans: a key intermediate bridge between basic and clinical sciences. Neuropsychopharmacology 44:1999–2010
Grupe DW, Nitschke JB (2013) Uncertainty and anticipatino in anxiety: an integrated neurobiological and psychological perspective. Nat Rev Neurosci 14:488–501
Grupe DW, Wielgosz J, Davidson RJ, Nitschke JB (2016) Neurobiological correlates of distinct post-traumatic stress disorder symptom profiles during threat anticipation in combat veterans. Psychol Med 46:1885–1895
Kaye JT, Bradford DE, Curtin JJ (2016) Psychometric properties of startle and corrugator response in NPU, affective picture viewing, and resting state tasks. Psychophysiology 53:1241–1255
Kaye JT, Fronk GE, Zgierska AE et al (2019) Acute prazosin administration does not reduce stressor reactivity in healthy adults. Psychopharmacology 236:3371–3382
Kesselheim AS, Hwang TJ, Franklin JM (2015) Two decades of new drug development for central nervous system disorders. Nat Rev Drug Discov 14:815–816
Klahn AL, Klinkenberg IAG, Notzon S et al (2016) Prepare for scare-impact of threat predictability on affective visual processing in spider phobia. Behav Brain Res 307:84–91
Klahn AL, Klinkenberg IA, Lueken U et al (2017) Commonalities and differences in the neural substrates of threat predictability in panic disorder and specific phobia. NeuroImage Clin 14:530–537
Krystal AD, Pizzagalli DA, Mathew SJ et al (2019) The first implementation of the NIMH FAST-FAIL approach to psychiatric drug development. Nat Rev Drug Discov 18:82–84
Lago TR, Hsiung A, Leitner BP et al (2018) Exercise decreases defensive responses to unpredictable, but not predictable, threat. Depress Anxiety 35:868–875
LeDoux JE, Pine DS (2016) Using neuroscience to help understand fear and anxiety: a two-system framework. Am J Psychiatry 173:1083–1093
Lee RJ, Coccaro EF, Cremers H et al (2013) A novel V1a receptor antagonist blocks vasopressin-induced changes in the CNS response to emotional stimuli: an fMRI study. Front Syst Neurosci 7:1–11
Lieberman L, Funkhouser CJ, Gorka SM et al (2020) The relation between posttraumatic stress symptom severity and startle potentiation to predictable and unpredictable threat. J Nerv Ment Dis 208(5):397–402
Lu Q, Lai J, Du Y et al (2019) Sexual dimorphism of oxytocin and vasopressin in social cognition and behavior. Psychol Res Behav Manag 12:337–349
Meyer-Lindenberg A, Kolachana B, Gold B et al (2009) Genetic variants in AVPR1A linked to autism predict amygdala activation and personality traits in healthy humans. Mol Psychiatry 14:968–975
Mineka S, Oehlberg K (2008) The relevance of recent developments in classical conditioning to understanding the etiology and maintenance of anxiety disorders. Acta Psychol (Amst) 127:567–580
Morgan CA III, Grillon C, Southwick SM et al (1995) Fear-potentiated startle in posttraumatic stress disorder. Biol Psychiatry 38:378–385
Peskind ER, Jensen CF, Pascualy M et al (1998) Sodium lactate and hypertonic sodium chloride induce equivalent panic incidence, panic symptoms, and hypernatremia in panic disorder. Biol Psychiatry 44:1007–1016
Pitman RK, Orr SP, Lasko NB (1993) Effects of intranasal vasopressin and oxytocin on physiologic responding during personal combat imagery in Vietnam veterans with posttraumatic stress disorder. Psychiatry Res 48:107–117
Pole N, Neylan TC, Best SR et al (2003) Fear-potentiated startle and posttraumatic stress symptoms in urban police officers. J Trauma Stress 16:471–479
Pole N, Neylan TC, Otte C et al (2009) Prospective prediction of posttraumatic stress disorder symptoms using fear potentiated auditory startle responses. Biol Psychiatry 65:235–240
Posner K, Brown GK, Stanley B et al (2011) The Columbia-Suicide Severity Rating Scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am J Psychiatry 168:1266–1277
Ring RH (2005) The central vasopressinergic system: examining the opportunities for psychiatric drug development. Curr Pharm Des 11:205–225
Robinson OJ, Overstreet C, Allen PS et al (2012) Acute tryptophan depletion increases translational indices of anxiety but not fear: serotonergic modulation of the bed nucleus of the stria terminalis? Neuropsychopharmacology 37:1963–1971
Rood BD, Beck SG (2014) Vasopressin indirectly excites dorsal raphe serotonin neurons through activation of the vasopressin1A receptor. Neuroscience 260:205–216
Ross AP, McCann KE, Larkin TE et al (2019) Sex-dependent effects of social isolation on the regulation of arginine-vasopressin (AVP) V1a, oxytocin (OT) and serotonin (5HT) 1a receptor binding and aggression. Horm Behav 116:1045678
Sartori SB, Singewald N (2019) Novel pharmacological targets in drug development for the treatment of anxiety and anxiety-related disorders. Pharmacol Ther 204:1–33
Schmitz A, Grillon C (2012) Assessing fear and anxiety in humans using the threat of predictable and unpredictable aversive events (the NPU-threat test). Nat Protoc 7:527–532
Shackman AJ, Fox AS (2016) Contributions of the central extended amygdala to fear and anxiety. J Neurosci 36:8050–8063
Thompson RR, George K, Walton JC et al (2006) Sex-specific influences of vasopressin on human social communication. PNAS 103:7889–7894
Wigger A, Sánchez MM, Mathys KC et al (2004) Alterations in central neuropeptide expression, release, and receptor binding in rats bred for high anxiety: critical role of vasopressin. Neuropsychopharmacology 29:1–14
Zinbarg RE, Barlow DH (1996) Structure of anxiety and the anxiety disorders: a hierarchical model. J Abnorm Psychol 105:181–193
Acknowledgements
We would like to thank Dr. Carlos Zarate, Jr. and Dr. Peixiong Yuan for assistance with plasma sample storage and shipping. We would like to thank NIMH nurses for their assistance with clinical coverage. We would like to thank Dr. Deborah Roberts for her assistance with protocol management.
Funding
This study was supported by the Intramural Research Program of the National Institute of Mental Health (grant number ZIAMH002798), Protocol 17-M-0046, NCT03036397 (clinicaltrials.gov). Azevan Pharmaceuticals Inc. provided SRX246 and placebo without charge and funded analysis of plasma samples for drug content. No NIH investigator involved in this study received any payment or other benefits from Azevan Pharmaceuticals Inc. T.L., E.P., E.B., A.M., A.B., C.R., N.B., E.D., S.P., M.E., and C.G. have nothing to disclose. M.B. and N.S. serve as officers at Azevan Pharmaceuticals and hold equity in the Company.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
T.L., E.P., E.B., A.M., A.B., C.R., N.B., E.D., S.P., M.E., and C.G. have nothing to disclose. M.B. and N.S. serve as officers at Azevan Pharmaceuticals Inc. and hold equity in the Company. Azevan Pharmaceuticals Inc. provided SRX246 and placebo without charge and funded analysis of plasma samples for drug content. T.L., E.P., E.B., A.M., A.B., C.R., N.B., S.P., M.E., and C.G. did not receive any payment or other benefits from Azevan Pharmaceuticals Inc.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lago, T.R., Brownstein, M.J., Page, E. et al. The novel vasopressin receptor (V1aR) antagonist SRX246 reduces anxiety in an experimental model in humans: a randomized proof-of-concept study. Psychopharmacology 238, 2393–2403 (2021). https://doi.org/10.1007/s00213-021-05861-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-021-05861-4