Abstract
Rationale
In rodents, exposure to novel environments elicits initial anxiety-like behavior (neophobia) followed by intense exploration (neophilia) that gradually subsides as the environment becomes familiar. Thus, innate novelty-induced behaviors are useful indices of anxiety and motivation in animal models of psychiatric disease. Noradrenergic neurons are activated by novelty and implicated in exploratory and anxiety-like responses, but the role of norepinephrine (NE) in neophobia has not been clearly delineated.
Objective
We sought to define the role of central NE transmission in neophilic and neophobic behaviors.
Methods
We assessed dopamine β-hydroxylase knockout (Dbh −/−) mice lacking NE and their NE-competent (Dbh +/−) littermate controls in neophilic (novelty-induced locomotion; NIL) and neophobic (novelty-suppressed feeding; NSF) behavioral tests with subsequent quantification of brain-wide c-fos induction. We complimented the gene knockout approach with pharmacological interventions.
Results
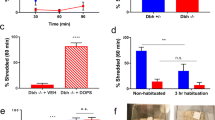
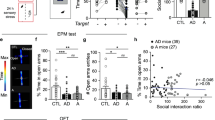
Dbh −/− mice exhibited blunted locomotor responses in the NIL task and completely lacked neophobia in the NSF test. Neophobia was rescued in Dbh −/− mice by acute pharmacological restoration of central NE with the synthetic precursor l-3,4-dihydroxyphenylserine (DOPS), and attenuated in control mice by the inhibitory α2-adrenergic autoreceptor agonist guanfacine. Following either NSF or NIL, Dbh −/− mice demonstrated reduced c-fos in the anterior cingulate cortex, medial septum, ventral hippocampus, bed nucleus of the stria terminalis, and basolateral amygdala.
Conclusion
These findings indicate that central NE signaling is required for the expression of both neophilic and neophobic behaviors. Further, we describe a putative noradrenergic novelty network as a potential therapeutic target for treating anxiety and substance abuse disorders.







Similar content being viewed by others
References
Angoa-Pérez M, Kane MJ, Briggs DI, Sykes CE, Shah MM, Francescutti DM, Rosenberg DR, Thomas DM, Kuhn DM (2012) Genetic depletion of brain 5HT reveals a common molecular pathway mediating compulsivity and impulsivity. J Neurochem 121:974–984
Archer T, Ogren S-O, Ross SB (1981) N-2-chloroethyl-N-ethyl-2-bromobenzylamine hydrochloride (DSP4), a new selective noradrenaline neurotoxin, and taste neophobia in the rat. Physiol Psychol 9:197–202
Aston-Jones G, Bloom F (1981) Nonrepinephrine-containing locus coeruleus neurons in behaving rats exhibit pronounced responses to non-noxious environmental stimuli. J Neurosci 1:887–900
Aston-Jones G, Cohen JD (2005) An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annu Rev Neurosci 28:403–450
Aston-Jones G, Iba M, Clayton E, Rajkowski J, Cohen J (2007) The locus coeruleus and regulation of behavioral flexibility and attention: clinical implications. In: Brain norepinephrine: Neurobiology, pp 196–235
Aston-Jones G, Kalivas PW (2008) Brain norepinephrine rediscovered in addiction research. Biol Psychiatry 63:1005–1006
Aston-Jones G, Rajkowski J, Cohen J (1999) Role of locus coeruleus in attention and behavioral flexibility. Biol Psychiatry 46:1309–1320
Aston-Jones G, Valentino RJ, Van Bockstaele EJ, Meyerson AT. (1994). Locus coeruleus, stress, and PTSD: neurobiological and clinical parallels
Avery S, Clauss J, Blackford J (2016) The human BNST: functional role in anxiety and addiction. Neuropsychopharmacology 41:126–141
Balderston NL, Schultz DH, Helmstetter FJ (2011) The human amygdala plays a stimulus specific role in the detection of novelty. Neuroimage 55:1889–1898
Bannerman D, Deacon R, Offen S, Friswell J, Grubb M, Rawlins J (2002) Double dissociation of function within the hippocampus: spatial memory and hyponeophagia. Behav Neurosci 116:884–901
Bannerman D, Matthews P, Deacon R, Rawlins J (2004) Medial septal lesions mimic effects of both selective dorsal and ventral hippocampal lesions. Behav Neurosci 118:1033–1041
Bardo MT, Donohew RL, Harrington NG (1996) Psychobiology of novelty seeking and drug seeking behavior. Behav Brain Res 77:23–43
Barnett SA (1958) Experiments on ‘neophobia’ in wild and laboratory rats. Br J Psychol 49:195–201
Beas BS, Wright BJ, Skirzewski M, Leng Y, Hyun JH, Koita O, Ringelberg N, Kwon H-B, Buonanno A, Penzo MA (2018) The locus coeruleus drives disinhibition in the midline thalamus via a dopaminergic mechanism. Nat Neurosci 21:963–973. https://doi.org/10.1038/s41593-018-0167-4
Bergado JA, Frey S, López J, Almaguer-Melian W, Frey JU (2007) Cholinergic afferents to the locus coeruleus and noradrenergic afferents to the medial septum mediate LTP-reinforcement in the dentate gyrus by stimulation of the amygdala. Neurobiol Learn Mem 88:331–341. https://doi.org/10.1016/j.nlm.2007.05.003
Berridge C, Espana R (2000) Synergistic sedative effects of noradrenergic α1-and β-receptor blockade on forebrain electroencephalographic and behavioral indices. Neuroscience 99:495–505
Berridge CW, Bolen SJ, Manley MS, Foote SL (1996) Modulation of forebrain electroencephalographic activity in halothane-anesthetized rat via actions of noradrenergic β-receptors within the medial septal region. J Neurosci 16:7010–7020
Berridge CW, Dunn AJ (1989) Restraint-stress-induced changes in exploratory behavior appear to be mediated by norepinephrine-stimulated release of CRF. J Neurosci 9:3513–3521
Blier P, El Mansari M (2007) The importance of serotonin and noradrenaline in anxiety. Int J Psychiatry Clin Pract 11:16–23
Boehnlein JK, Kinzie JD (2007) Pharmacologic reduction of CNS noradrenergic activity in PTSD: the case for clonidine and prazosin. J Psychiatr Pract 13:72–78
Bonnavion P, Mickelsen LE, Fujita A, Lecea L, Jackson AC (2016) Hubs and spokes of the lateral hypothalamus: cell types, circuits and behaviour. J Physiol 594:6443–6462
Bourdélat-Parks BN, Anderson GM, Donaldson ZR, Weiss JM, Bonsall RW, Emery MS, Liles LC, Weinshenker D (2005) Effects of dopamine β-hydroxylase genotype and disulfiram inhibition on catecholamine homeostasis in mice. Psychopharmacology 183:72–80
Brady LS (1994) Stress, antidepressant drugs, and the locus coeruleus. Brain Res Bull 35:545–556
Britton DR, Indyk E (1990) Central effects of corticotropin releasing factor (CRF): evidence for similar interactions with environmental novelty and with caffeine. Psychopharmacology 101:366–370
Brown SP, Mathur BN, Olsen SR, Luppi P-H, Bickford ME, Citri A (2017) New breakthroughs in understanding the role of functional interactions between the neocortex and the claustrum. J Neurosci 37:10877–10881. https://doi.org/10.1523/JNEUROSCI.1837-17.2017
Buffalari DM, Grace AA (2007) Noradrenergic modulation of basolateral amygdala neuronal activity: opposing influences of α-2 and β receptor activation. J Neurosci 27:12358–12366
Carpenter F, Burgess N, Barry C (2017) Modulating medial septal cholinergic activity reduces medial entorhinal theta frequency without affecting speed or grid coding. Sci Rep 7:14573
Chandler DJ, Gao W-J, Waterhouse BD (2014) Heterogeneous organization of the locus coeruleus projections to prefrontal and motor cortices. Proc Natl Acad Sci 111:6816–6821
Chandler DJ (2016) Evidence for a specialized role of the locus coeruleus noradrenergic system in cortical circuitries and behavioral operations. Brain Res 1641:197–206
Chen Y-W, Das M, Oyarzabal EA, Cheng Q, Plummer NW, Smith KG, Jones GK, Malawsky D, Yakel JL, Shih Y-YI, Jensen P (2019) Genetic identification of a population of noradrenergic neurons implicated in attenuation of stress-related responses. Mol Psychiatry 24:710–725. https://doi.org/10.1038/s41380-018-0245-8
Chiang MC, Bowen A, Schier LA, Tupone D, Uddin O, Heinricher MM (2019) Parabrachial complex: a hub for pain and aversion. J Neurosci 39:8225–8230. https://doi.org/10.1523/JNEUROSCI.1162-19.2019
Choi EA, McNally GP (2017) Paraventricular thalamus balances danger and reward. J Neurosci 37:3018–3029
Crick FC, Koch C (2005) What is the function of the claustrum? Philosophical transactions of the Royal Society of London. Series B Biologic Sci 360:1271–1279. https://doi.org/10.1098/rstb.2005.1661
Cryan JF, Sweeney FF (2011) The age of anxiety: role of animal models of anxiolytic action in drug discovery. Br J Pharmacol 164:1129–1161
Cubells JF, Schroeder JP, Barrie ES, Manvich DF, Sadee W, Berg T, Mercer K, Stowe TA, Liles LC, Squires KE, Mezher A, Curtin P, Perdomo DL, Szot P, Weinshenker D (2016) Human bacterial artificial chromosome (BAC) transgenesis fully rescues noradrenergic function in dopamine β-hydroxylase knockout mice. PLoS One 11:e0154864. https://doi.org/10.1371/journal.pone.0154864
Dabrowska J, Martinon D, Moaddab M, Rainnie DG (2016) Targeting corticotropin-releasing factor projections from the oval nucleus of the bed nucleus of the stria terminalis using cell-type specific neuronal tracing studies in mouse and rat brain. J Neuroendocrinol 28:10. https://doi.org/10.1111/jne.12442
Delini-Stula A, Mogilnicka E, Hunn C, Dooley D (1984) Novelty-oriented behavior in the rat after selective damage of locus coeruleus projections by DSP-4, a new noradrenergic neurotoxin. Pharmacol Biochem Behav 20:613–618
Dovey TM, Staples PA, Gibson EL, Halford JCG (2008) Food neophobia and ‘picky/fussy’ eating in children: a review. Appetite 50:181–193
Dulawa SC (2009) Novelty-induced hypophagia. In: Mood and anxiety related phenotypes in mice. Springer, Berlin, pp 247–259
Dulawa SC, Hen R (2005) Recent advances in animal models of chronic antidepressant effects: the novelty-induced hypophagia test. Neurosci Biobehav Rev 29:771–783
Egli RE, Kash TL, Choo K, Savchenko V, Matthews RT, Blakely RD, Winder DG (2005) Norepinephrine modulates glutamatergic transmission in the bed nucleus of the stria terminalis. Neuropsychopharmacology 30:657–668
Egner T (2011) Surprise! A unifying model of dorsal anterior cingulate function? Nat Neurosci 14:1219–1220
Fanselow MS, Dong H-W (2010) Are the dorsal and ventral hippocampus functionally distinct structures? Neuron 65:7–19. https://doi.org/10.1016/j.neuron.2009.11.031
File SE (2001) Factors controlling measures of anxiety and responses to novelty in the mouse. Behav Brain Res 125:151–157
Fox HC, Seo D, Tuit K, Hansen J, Kimmerling A, Morgan PT, Sinha R (2012) Guanfacine effects on stress, drug craving and prefrontal activation in cocaine dependent individuals: preliminary findings. J Psychopharmacol 26:958–972
Gehrlach DA, Dolensek N, Klein AS, Roy Chowdhury R, Matthys A, Junghänel M, Gaitanos TN, Podgornik A, Black TD, Reddy Vaka N, Conzelmann K-K, Gogolla N (2019) Aversive state processing in the posterior insular cortex. Nat Neurosci 22:1424–1437. https://doi.org/10.1038/s41593-019-0469-1
Goddard AW, Ball SG, Martinez J, Robinson MJ, Yang CR, Russell JM, Shekhar A (2010) Current perspectives of the roles of the central norepinephrine system in anxiety and depression. Depress Anxiety 27:339–350
Gompf HS, Mathai C, Fuller PM, Wood DA, Pedersen NP, Saper CB, Lu J (2010) Locus ceruleus and anterior cingulate cortex sustain wakefulness in a novel environment. J Neurosci 30:14543–14551
Guiard BP, El Mansari M, Blier PJ. (2008). Crosstalk between dopaminergic and noradrenergic systems in the rat ventral tegmental area, locus coeruleus, and dorsal hippocampus
Harro J, Oreland L, Vasar E, Bradwejn JJEN (1995) Impaired exploratory behaviour after DSP-4 treatment in rats: implications for the increased anxiety after noradrenergic denervation. Eur Neuropsychopharmacol 5:447–455
Hoehn-Saric R, Merchant AF, Keyser ML (1981) Effects of clonidine on anxiety disorders. Arch Gen Psychiatry 38:1278–1282
Jhang J, Lee H, Kang MS, Lee H-S, Park H, Han J-H (2018) Anterior cingulate cortex and its input to the basolateral amygdala control innate fear response. Nat Commun 9:2744
Jiang Y-Y, Zhang Y, Cui S, Liu F-Y, Yi M, Wan Y (2018) Cholinergic neurons in medial septum maintain anxiety-like behaviors induced by chronic inflammatory pain. Neurosci Lett 671:7–12. https://doi.org/10.1016/j.neulet.2018.01.041
Jimenez JC, Su K, Goldberg AR, Luna VM, Biane JS, Ordek G, Zhou P, Ong SK, Wright MA, Zweifel L (2018) Anxiety cells in a hippocampal-hypothalamic circuit. Neuron 97:670–683 e676
Kabbaj M, Devine DP, Savage VR, Akil H (2000) Neurobiological correlates of individual differences in novelty-seeking behavior in the rat: differential expression of stress-related molecules. J Neurosci 20:6983–6988
Kafkas A, Montaldi D (2014) Two separate, but interacting, neural systems for familiarity and novelty detection: a dual-route mechanism. Hippocampus 24:516–527
Kafkas A, Montaldi D (2018) How do memory systems detect and respond to novelty? Neurosci Lett 680:60–68. https://doi.org/10.1016/j.neulet.2018.01.053
Kalueff A, Wheaton M, Murphy D (2007) What’s wrong with my mouse model?: advances and strategies in animal modeling of anxiety and depression. Behav Brain Res 179:1–18
Kempadoo KA, Mosharov EV, Choi SJ, Sulzer D, Kandel ER (2016) Dopamine release from the locus coeruleus to the dorsal hippocampus promotes spatial learning and memory. Proc Natl Acad Sci 113:14835–14840
Kirouac GJ (2015) Placing the paraventricular nucleus of the thalamus within the brain circuits that control behavior. Neurosci Biobehav Rev 56:315–329. https://doi.org/10.1016/j.neubiorev.2015.08.005
Klumpp H, Angstadt M, Phan KL (2012) Insula reactivity and connectivity to anterior cingulate cortex when processing threat in generalized social anxiety disorder. Biol Psychol 89:273–276
Koob GF, Simon EJ (2009) The neurobiology of addiction: where we have been and where we are going. J Drug Issues 39:115–132. https://doi.org/10.1177/002204260903900110
Koob GF, Volkow ND (2016) Neurobiology of addiction: a neurocircuitry analysis. Lancet Psychiatry 3:760–773. https://doi.org/10.1016/S2215-0366(16)00104-8
Lebow MA, Chen A (2016) Overshadowed by the amygdala: the bed nucleus of the stria terminalis emerges as key to psychiatric disorders. Mol Psychiatry 21:450–463. https://doi.org/10.1038/mp.2016.1
Li L, Wang L (2018) Modulation of innate defensive responses by locus coeruleus-superior colliculus circuit. J Exper Neurosci 12:1179069518792035
Lindeberg J, Usoskin D, Bengtsson H, Gustafsson A, Kylberg A, Söderström S, Ebendal T (2004) Transgenic expression of Cre recombinase from the tyrosine hydroxylase locus. Genesis 40:67–73. https://doi.org/10.1002/gene.20065
Llorca-Torralba M, Suárez-Pereira I, Bravo L, Camarena-Delgado C, Garcia-Partida JA, Mico JA, Berrocoso E (2019) Chemogenetic silencing of the locus coeruleus–basolateral amygdala pathway abolishes pain-induced anxiety and enhanced aversive learning in rats. Biol Psychiatry 85:1021–1035
Lustberg D, Iannitelli AF, Tillage RP, Pruitt M, Liles LC, Weinshenker D (2020) Central norepinephrine transmission is required for stress-induced repetitive behavior in two rodent models of obsessive-compulsive disorder. Psychopharmacology 237:1973–1987
Mantsch JR, Baker DA, Funk D, Lê AD, Shaham Y (2016) Stress-induced reinstatement of drug seeking: 20 years of progress. Neuropsychopharmacology 41:335–356. https://doi.org/10.1038/npp.2015.142
Marazziti D, Carlini M, Dell’Osso L (2012) Treatment strategies of obsessive-compulsive disorder and panic disorder/agoraphobia. Curr Top Med Chem 12:238–253
Marcontell DK, Laster AE, Johnson J (2003) Cognitive-behavioral treatment of food neophobia in adults. J Anxiety Disord 17:243–251
Marino MD, Bourdélat-Parks BN, Liles LC, Weinshenker D (2005) Genetic reduction of noradrenergic function alters social memory and reduces aggression in mice. Behav Brain Res 161:197–203
Martin EI, Ressler KJ, Binder E, Nemeroff CB (2009) The neurobiology of anxiety disorders: brain imaging, genetics, and psychoneuroendocrinology. Psychiatric Clin North Am 32:549–575. https://doi.org/10.1016/j.psc.2009.05.004
Mather M, Clewett D, Sakaki M, Harley CW (2016) Norepinephrine ignites local hotspots of neuronal excitation: how arousal amplifies selectivity in perception and memory. The Behavioral and brain sciences 39:e200–e200. https://doi.org/10.1017/S0140525X15000667
McCall JG, Al-Hasani R, Siuda ER, Hong DY, Norris AJ, Ford CP, Bruchas MR (2015) CRH engagement of the locus coeruleus noradrenergic system mediates stress-induced anxiety. Neuron 87:605–620
McCall JG, Siuda ER, Bhatti DL, Lawson LA, McElligott ZA, Stuber GD, Bruchas MR (2017) Locus coeruleus to basolateral amygdala noradrenergic projections promote anxiety-like behavior. eLife 6:e18247. https://doi.org/10.7554/eLife.18247
Mineur YS, Bentham MP, Zhou W-L, Plantenga ME, McKee SA, Picciotto MR (2015) Antidepressant-like effects of guanfacine and sex-specific differences in effects on c-fos immunoreactivity and paired-pulse ratio in male and female mice. Psychopharmacology 232:3539–3549
Mitchell HA, Ahern TH, Liles LC, Javors MA, Weinshenker D (2006) The effects of norepinephrine transporter inactivation on locomotor activity in mice. Biol Psychiatry 60:1046–1052
Mitchell HA, Bogenpohl JW, Liles LC, Epstein MP, Bozyczko-Coyne D, Williams M, Weinshenker D (2008) Behavioral responses of dopamine β-hydroxylase knockout mice to modafinil suggest a dual noradrenergic–dopaminergic mechanism of action. Pharmacol Biochem Behav 91:217–222
Modlinska K, Stryjek R (2016) Food Neophobia in wild rats (Rattus norvegicus) inhabiting a changeable environment-a field study. PLoS One 11:e0156741–e0156741. https://doi.org/10.1371/journal.pone.0156741
Modlinska K, Stryjek R, Pisula W (2015) Food neophobia in wild and laboratory rats (multi-strain comparison). Behav Process 113:41–50
Mosienko V, Bert B, Beis D, Matthes S, Fink H, Bader M, Alenina N (2012) Exaggerated aggression and decreased anxiety in mice deficient in brain serotonin. Transl Psychiatry 2:e122–e122
Murchison CF, Zhang X-Y, Zhang W-P, Ouyang M, Lee A, Thomas SA (2004) A distinct role for norepinephrine in memory retrieval. Cell 117:131–143
Myhrer T (1989) Exploratory behavior and reaction to novelty in rats: effects of medial and lateral septal lesions. Behav Neurosci 103:1226–1233
Naqvi NH, Bechara A (2010) The insula and drug addiction: an interoceptive view of pleasure, urges, and decision-making. Brain Struct Funct 214:435–450
Neophytou SI, Aspley S, Butler S, Beckett S, Marsden CA. (2001). Effects of lesioning noradrenergic neurones in the locus coeruleus on conditioned and unconditioned aversive behaviour in the rat 25:1307–1321
Olson VG, Heusner CL, Bland RJ, During MJ, Weinshenker D, Palmiter RD (2006) Role of noradrenergic signaling by the nucleus tractus solitarius in mediating opiate reward. Science 311:1017–1020. https://doi.org/10.1126/science.1119311
Osorio-Gómez D, Guzmán-Ramos K, Bermúdez-Rattoni F (2018) Neurobiology of neophobia and its attenuation. In: Food neophobia. Elsevier, Berlin, pp 111–128
Padilla-Coreano N, Bolkan SS, Pierce GM, Blackman DR, Hardin WD, Garcia-Garcia AL, Spellman TJ, Gordon JA (2016) Direct ventral hippocampal-prefrontal input is required for anxiety-related neural activity and behavior. Neuron 89:857–866. https://doi.org/10.1016/j.neuron.2016.01.011
Pawlak CR, Ho Y-J, Schwarting RKW (2008) Animal models of human psychopathology based on individual differences in novelty-seeking and anxiety. Neurosci Biobehav Rev 32:1544–1568
Petrides M (2007) The orbitofrontal cortex: novelty, deviation from expectation, and memory. Ann N Y Acad Sci 1121:33–53
Poletti S, Radaelli D, Cucchi M, Ricci L, Vai B, Smeraldi E, Benedetti F (2015) Neural correlates of anxiety sensitivity in panic disorder: a functional magnetic resonance imaging study. Psychiatry Res Neuroimaging 233:95–101
Porter-Stransky KA, Centanni SW, Karne SL, Odil LM, Fekir S, Wong JC, Jerome C, Mitchell HA, Escayg A, Pedersen NP (2019) Noradrenergic transmission at Alpha1-adrenergic receptors in the ventral periaqueductal gray modulates arousal. Biol Psychiatry 85:237–247
Qadir H, Krimmel SR, Mu C, Poulopoulos A, Seminowicz DA, Mathur BN (2018) Structural connectivity of the anterior cingulate cortex, claustrum, and the anterior insula of the mouse. Front Neuroanat 12:100
Ricardo JA, Koh ET (1978) Anatomical evidence of direct projections from the nucleus of the solitary tract to the hypothalamus, amygdala, and other forebrain structures in the rat. Brain Res 153:1–26
Rinaldi A, Romeo S, Agustin-Pavon C, Oliverio A, Mele A (2010) Distinct patterns of Fos immunoreactivity in striatum and hippocampus induced by different kinds of novelty in mice. Neurobiol Learn Mem 94:373–381
Rinaman L (2011) Hindbrain noradrenergic A2 neurons: diverse roles in autonomic, endocrine, cognitive, and behavioral functions. Am J Physiol Regul Integr Comp Physiol 300:R222–R235. https://doi.org/10.1152/ajpregu.00556.2010
Robertson SD, Plummer NW, de Marchena J, Jensen P (2013) Developmental origins of central norepinephrine neuron diversity. Nat Neurosci 16:1016–1023. https://doi.org/10.1038/nn.3458
Robertson SD, Plummer NW, Jensen P (2016) Uncovering diversity in the development of central noradrenergic neurons and their efferents. Brain Res 1641:234–244. https://doi.org/10.1016/j.brainres.2015.11.023
Rojas S, Diaz-Galarce R, Jerez-Baraona JM, Quintana-Donoso D, Moraga-Amaro R, Stehberg J (2015) The insula modulates arousal-induced reluctance to try novel tastes through adrenergic transmission in the rat. Front Behav Neurosci 9:164
Rolls ET, Grabenhorst F (2008) The orbitofrontal cortex and beyond: from affect to decision-making. Prog Neurobiol 86:216–244
Rommelfanger K, Edwards G, Freeman K, Liles L, Miller G, Weinshenker D (2007) Norepinephrine loss produces more profound motor deficits than MPTP treatment in mice. Proc Natl Acad Sci 104:13804–13809
Sadacca BF, Wikenheiser AM, Schoenbaum G (2017) Toward a theoretical role for tonic norepinephrine in the orbitofrontal cortex in facilitating flexible learning. Neuroscience 345:124–129
Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, Weisstaub N, Lee J, Duman R, Arancio O (2003) Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science 301:805–809
Sara Susan J, Bouret S (2012) Orienting and reorienting: the locus coeruleus mediates cognition through arousal. Neuron 76:130–141. https://doi.org/10.1016/j.neuron.2012.09.011
Sara SJ, Dyon-Laurent C, Hervé A (1995) Novelty seeking behavior in the rat is dependent upon the integrity of the noradrenergic system. Cogn Brain Res 2:181–187
Savitt JM, Jang SS, Mu W, Dawson VL, Dawson TM (2005) Bcl-x is required for proper development of the mouse substantia nigra. J Neurosci 25:6721–6728
Schank JR, Liles LC, Weinshenker D (2008) Norepinephrine signaling through β-adrenergic receptors is critical for expression of cocaine-induced anxiety. Biol Psychiatry 63:1007–1012
Schank JR, Ventura R, Puglisi-Allegra S, Alcaro A, Cole CD, Liles LC, Seeman P, Weinshenker D (2006) Dopamine β-hydroxylase knockout mice have alterations in dopamine signaling and are hypersensitive to cocaine. Neuropsychopharmacology 31:2221–2230
Scholtysik G (1980) Pharmacology of guanfacine. Br J Clin Pharmacol 10:21S–24S
Schroeder JP, Epps SA, Grice TW, Weinshenker D (2013) The selective dopamine β-hydroxylase inhibitor nepicastat attenuates multiple aspects of cocaine-seeking behavior. Neuropsychopharmacology 38:1032–1038
Schwarz LA, Luo L (2015) Organization of the locus coeruleus-norepinephrine system. Curr Biol 25:R1051–R1056
Schwarz LA, Miyamichi K, Gao XJ, Beier KT, Weissbourd B, DeLoach KE, Ren J, Ibanes S, Malenka RC, Kremer EJ, Luo L (2015) Viral-genetic tracing of the input–output organization of a central noradrenaline circuit. Nature 524:88–92. https://doi.org/10.1038/nature14600
Sheth A, Berretta S, Lange N, Eichenbaum H (2008) The amygdala modulates neuronal activation in the hippocampus in response to spatial novelty. Hippocampus 18:169–181
Siuda ER, Al-Hasani R, McCall JG, Bhatti DL, Bruchas MR (2016) Chemogenetic and optogenetic activation of Gαs signaling in the basolateral amygdala induces acute and social anxiety-like states. Neuropsychopharmacol : off publicat Am College Neuropsychopharmacol 41:2011–2023. https://doi.org/10.1038/npp.2015.371
Snyder K, Wang W-W, Han R, McFadden K, Valentino RJ (2012) Corticotropin-releasing factor in the norepinephrine nucleus, locus coeruleus, facilitates behavioral flexibility. Neuropsychopharmacology 37:520–530
Soya S, Takahashi TM, McHugh TJ, Maejima T, Herlitze S, Abe M, Sakimura K, Sakurai T (2017) Orexin modulates behavioral fear expression through the locus coeruleus. Nat Commun 8:1606
Stachniak TJ, Ghosh A, Sternson SM (2014) Chemogenetic synaptic silencing of neural circuits localizes a hypothalamus → midbrain pathway for feeding behavior. Neuron 82:797–808
Stone E, Zhang Y, Rosengarten H, Yeretsian J, Quartermain D (1999) Brain alpha 1-adrenergic neurotransmission is necessary for behavioral activation to environmental change in mice. Neuroscience 94:1245–1252
Stone EA, Lin Y, Ahsan MR, Quartermain D (2005) α1-adrenergic and α2-adrenergic balance in the dorsal pons and gross behavioral activity of mice in a novel environment. Psychopharmacology 183:127
Struthers WM, DuPriest A, Runyan J (2005) Habituation reduces novelty-induced FOS expression in the striatum and cingulate cortex. Exp Brain Res 167:136–140
Takeuchi T, Duszkiewicz AJ, Sonneborn A, Spooner PA, Yamasaki M, Watanabe M, Smith CC, Fernández G, Deisseroth K, Greene RW (2016) Locus coeruleus and dopaminergic consolidation of everyday memory. Nature 537:357–362
Tanaka M, Yoshida M, Emoto H, Ishii H (2000) Noradrenaline systems in the hypothalamus, amygdala and locus coeruleus are involved in the provocation of anxiety: basic studies. Eur J Pharmacol 405:397–406
Tereshchenko Y, Brandewiede J, Schachner M, Irintchev A, Morellini F (2008) Novelty-induced behavioral traits correlate with numbers of brainstem noradrenergic neurons and septal cholinergic neurons in C57BL/6J mice. Behav Brain Res 191:280–284
Thomas SA, Marck BT, Palmiter RD, Matsumoto AM (1998) Restoration of norepinephrine and reversal of phenotypes in mice lacking dopamine β-hydroxylase. J Neurochem 70:2468–2476
Thomas SA, Matsumoto AM, Palmiter RD (1995) Noradrenaline is essential for mouse fetal development. Nature 374:643–646
Thomas SA, Palmiter RD (1997) Disruption of the dopamine Β-hydroxylase gene in mice suggests roles for norepinephrine in motor function, learning, and memory. Behav Neurosci 111:579–589
Tillage RP, Sciolino NR, Plummer NW, Lustberg D, Liles LC, Hsiang M, Powell JM, Smith KG, Jensen P, Weinshenker D (2020) Elimination of galanin synthesis in noradrenergic neurons reduces galanin in select brain areas and promotes active coping behaviors. Brain Struct Funct:1–19
Tovote P, Fadok JP, Lüthi A (2015) Neuronal circuits for fear and anxiety. Nat Rev Neurosci 16:317–331
Tulving E, Markowitsch HJ, Kapur S, Habib R, Houle S (1994) Novelty encoding networks in the human brain: positron emission tomography data. Neuroreport 5:2525–2528
Uddin LQ, Nomi JS, Hebert-Seropian B, Ghaziri J, Boucher O (2017) Structure and function of the human insula. J Clin neurophysiol: official publicat Am Electroencephalograph Soc 34:300–306
Uematsu A, Tan BZ, Johansen JP (2015) Projection specificity in heterogeneous locus coeruleus cell populations: implications for learning and memory. Learning & memory (Cold Spring Harbor, NY) 22:444–451. https://doi.org/10.1101/lm.037283.114
Valentino RJ, Foote SL, Page ME (1993) The locus coeruleus as a site for integrating corticotropin-releasing factor and noradrenergic mediation of stress responses a. Ann N Y Acad Sci 697:173–188
van der Kooy D, Koda LY, McGinty JF, Gerfen CR, Bloom FE (1984) The organization of projections from the cortes, amygdala, and hypothalamus to the nucleus of the solitary tract in rat. J Comp Neurol 224:1–24
Vankov A, Hervé-Minvielle A, Sara SJ (1995) Response to novelty and its rapid habituation in locus coeruleus neurons of the freely exploring rat. Eur J Neurosci 7:1180–1187
Vranjkovic O, Pina M, Kash TL, Winder DG (2017) The bed nucleus of the stria terminalis in drug-associated behavior and affect: a circuit-based perspective. Neuropharmacology 122:100–106
Wagatsuma A, Okuyama T, Sun C, Smith LM, Abe K, Tonegawa S (2018) Locus coeruleus input to hippocampal CA3 drives single-trial learning of a novel context. Proc Natl Acad Sci 115:E310–E316
Walker QD, Schramm-Sapyta NL, Caster JM, Waller ST, Brooks MP, Kuhn CM (2009) Novelty-induced locomotion is positively associated with cocaine ingestion in adolescent rats; anxiety is correlated in adults. Pharmacol Biochem Behav 91:398–408
Weierich MR, Wright CI, Negreira A, Dickerson BC, Barrett LF (2010) Novelty as a dimension in the affective brain. Neuroimage 49:2871–2878
Weinshenker D, Miller NS, Blizinsky K, Laughlin ML, Palmiter RD (2002a) Mice with chronic norepinephrine deficiency resemble amphetamine-sensitized animals. Proc Natl Acad Sci 99:13873–13877
Weinshenker D, Schroeder JP (2007) There and back again: a tale of norepinephrine and drug addiction. Neuropsychopharmacology 32:1433–1451
Weinshenker D, White SS, Javors MA, Palmiter RD, Szot P (2002b) Regulation of norepinephrine transporter abundance by catecholamines and desipramine in vivo. Brain Res 946:239–246. https://doi.org/10.1016/S0006-8993(02)02889-5
West CHK, Ritchie JC, Boss-Williams KA, Weiss JM (2009) Antidepressant drugs with differing pharmacological actions decrease activity of locus coeruleus neurons. Int J Neuropsychopharmacol 12:627–641. https://doi.org/10.1017/S1461145708009474
Wingo T, Nesil T, Choi J-S, Li MD (2016) Novelty seeking and drug addiction in humans and animals: from behavior to molecules. J NeuroImmune Pharmacol 11:456–470
Wittmann A, Schlagenhauf F, Guhn A, Lueken U, Gaehlsdorf C, Stoy M, Bermpohl F, Fydrich T, Pfleiderer B, Bruhn H (2014) Anticipating agoraphobic situations: the neural correlates of panic disorder with agoraphobia. Psychol Med 44:2385–2396
Woon EP, Sequeira MK, Barbee BR, Gourley SL (2019) Involvement of the rodent prelimbic and medial orbitofrontal cortices in goal-directed action: a brief review. J Neurosci Res 98:1020–1030. https://doi.org/10.1002/jnr.24567
Zaborszky L (1989) Afferent connections of the forebrain cholinergic projection neurons, with special reference to monoaminergic and peptidergic fibers. EXS 57:12–32. https://doi.org/10.1007/978-3-0348-9138-7_2
Zhang Y, Jiang Y-Y, Shao S, Zhang C, Liu F-Y, Wan Y, Yi M (2017) Inhibiting medial septal cholinergic neurons with DREADD alleviated anxiety-like behaviors in mice. Neurosci Lett 638:139–144
Acknowledgments
We thank Lundbeck for providing the DOPS.
Funding
This work was supported by the National Institutes of Health (AG061175, NS102306, and DA038453 to DW; GM8602-22 to DL; MH116622 to RPT).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All animal procedures and protocols were congruent with the National Institutes of Health guidelines for the care and use of laboratory animals and were approved by the Emory University Animal Care and Use Committee.
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Fig. S1
Compsarison of c-fos induction under naïve conditions and after exposure to novelty. a Under behaviorally naïve conditions, c-fos induction was negligible and did not differ between genotypes any forebrain targets tested, including ventral hippocampal subfield CA1 (vCA1), anterior cingulate cortex (ACC), dorsal bed nucleus of the stria terminalis (dBNST), medial septum/diagonal band complex (MS/DB), and basolateral amygdala (BLA). Representative micrographs show low regional c-fos (green) induction in behaviorally naïve Dbh +/- control mice (top row) and Dbh -/- mice (bottom row). Nuclei were counterstained with DAPI (blue). b Representative micrographs showing c-fos induction in LC, ACC, and vCA1 of Dbh +/- control mice that were behaviorally naïve (top row) compared to those that were euthanized 90 min after exposure to the novel environment in the NIL test (bottom row). The scale bar denotes 100 μM in all micrographs. (PNG 3493 kb)
Rights and permissions
About this article
Cite this article
Lustberg, D., Tillage, R.P., Bai, Y. et al. Noradrenergic circuits in the forebrain control affective responses to novelty. Psychopharmacology 237, 3337–3355 (2020). https://doi.org/10.1007/s00213-020-05615-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-020-05615-8