Abstract
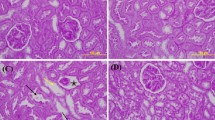
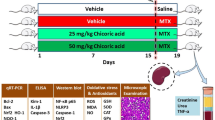
Methotrexate-induced nephrotoxicity is a medical emergency which is associated with a variety of side effects. Vanillic acid (VA), as an antioxidant, removes free radical oxygen to protect cell defense. Therefore, this study investigated VA’s beneficial effects on nephrotoxicity induced by methotrexate through its anti-apoptosis, antioxidant, and anti-inflammatory properties. Our study included five groups of male Wistar rats (n = 8): sham, MTX (Methotrexate) group: rats receiving methotrexate (20 mg/kg, intraperitoneally) on Day 2. Moreover, the remaining groups consisted of animals that received vanillic acid (25, 50, and 100 mg/kg, orally for seven days) plus MTX on the 2nd day. The rats were deeply anesthetized on the eighth day to obtain blood and renal tissue samples. The results showed that MTX can increase blood urea nitrogen and creatinine. However, VA (50 and 100 mg/kg) improved renal function as approved by histological findings. Compared with MTX-treated rats, VA enhanced the contents of total antioxidant capacity (TAC) and reduced renal malondialdehyde (MDA). Moreover, VA reduced mRNA expressions of caspase-3 and Bcl-2-associated x protein (Bax) and caused mRNA overexpression of the renal B-cell lymphoma-2 (Bcl-2), and Nrf-2 (Nuclear factor erythroid 2-related factor 2) compared to the MTX group. Also, VA administration significantly reduced inflammatory agents. Overall, VA protects the kidneys against methotrexate-induced nephrotoxicity via anti-apoptosis, antioxidant, and anti-inflammatory properties. Our results revealed that the most effective dose of VA was 100 mg/kg.





Similar content being viewed by others
Data availability
All data generated or analyzed during the current study are available from the corresponding author upon reasonable request.
Abbreviations
- VA:
-
Vanillic acid
- MTX:
-
Methotrexate
- TAC:
-
Total antioxidant capacity
- MDA:
-
Malondialdehyde
- BAX:
-
Bcl-2-associated x protein
- Bcl-2:
-
B-cell lymphoma-2
- TNF-α:
-
Tumor necrosis factor-α
- IL-1β:
-
Interleukine-1β
- NS:
-
Normal saline
- BUN:
-
Blood urea nitrogen
- Cr:
-
Creatinine
- qRT‑PCR:
-
Quantitative real‑time PCR
- H&E:
-
Hematoxylin-eosin
- ANOVA:
-
One-way analysis of variance
- NF-κβ:
-
Nuclear factor-κB
- GAPDH:
-
Glyceraldehyde-3-phosphate dehydrogenase
- Nrf-2:
-
Nuclear factor erythroid 2-related factor 2
References
Amini N, Badavi M, Mard SA, Dianat M, Moghadam MT (2022) The renoprotective effects of gallic acid on cisplatin-induced nephrotoxicity through anti-apoptosis, anti-inflammatory effects, and downregulation of lncRNA TUG1. Naunyn-Schmiedeberg’s Arch Pharmacol 395(6):691–701
Bai J, Zhang Y, Tang C, Hou Y, Ai X, Chen X, Zhang Y, Wang X, Meng X (2021) Gallic acid: Pharmacological activities and molecular mechanisms involved in inflammation-related diseases. Biomed Pharmacother 133:110985. https://doi.org/10.1016/j.biopha.2020.110985
Basile DP, Anderson MD, Sutton TA (2012) Pathophysiology of acute kidney injury. Compr Physiol 2(2):1303–1353
Chowdhury S, Sinha K, Banerjee S, Sil PC (2016) Taurine protects cisplatin induced cardiotoxicity by modulating inflammatory and endoplasmic reticulum stress responses. BioFactors 42(6):647–664
Dalle-Donne I, Rossi R, Colombo R, Giustarini D, Milzani A (2006) Biomarkers of oxidative damage in human disease. Clin Chem 52(4):601–623
Devrim E, Çetin R, Kılıçoğlu B, Imge Ergüder B, Avcı A, Durak İ (2005) Methotrexate causes oxidative stress in rat kidney tissues. Ren Fail 27:771–773
Elmore S (2007) Apoptosis: a review of programmed cell death. Toxicol Pathol 35:495–516
Gur C, Kandemir FM, Caglayan C, Satıcı E (2022) Chemopreventive effects of hesperidin against paclitaxel-induced hepatotoxicity and nephrotoxicity via amendment of Nrf2/HO-1 and caspase-3/Bax/Bcl-2 signaling pathways. Chem Biol Interact 365:110073. https://doi.org/10.1016/j.cbi.2022.110073. (Epub 2022 Jul 31)
Hassanein EHM, Shalkami AS, Khalaf MM, Mohamed WR, Hemeida RAM (2019) The impact of Keap1/Nrf2, P(38)MAPK/NF-κB and Bax/Bcl2/caspase-3 signaling pathways in the protective effects of berberine against methotrexate-induced nephrotoxicity. Biomed Pharmacother 109:47–56
Havasi A, Borkan SC (2011) Apoptosis and acute kidney injury. Kidney Int 80:29–40
Jaiswal AK (2004) Nrf2 signaling in coordinated activation of antioxidant gene expression. Free Radic Biol Med 36:1199–1207
Joardar S, Dewanjee S, Bhowmick S, Dua TK, Das S, Saha A, De Feo V (2019) Rosmarinic acid attenuates cadmium-induced nephrotoxicity via inhibition of oxidative stress, apoptosis, inflammation and fibrosis. Int J Mol Sci 20:2027
Kandemir FM, Kucukler S, Caglayan C, Gur C, Batil AA, Gülçin İ (2017) Therapeutic effects of silymarin and naringin on methotrexate-induced nephrotoxicity in rats: Biochemical evaluation of anti-inflammatory, antiapoptotic, and antiautophagic properties. J Food Biochem 41:e12398. https://doi.org/10.1111/jfbc.12398
Kiokias S, Proestos C, Oreopoulou V (2020) Phenolic acids of plant origin—A review on their antioxidant activity in vitro (o/w emulsion systems) along with their in vivo health biochemical properties. Foods 9:534. https://doi.org/10.3390/foods9040534
Liu M, Grigoryev DN, Crow MT, Haas M, Yamamoto M, Reddy SP, Rabb H (2009) Transcription factor Nrf2 is protective during ischemic and nephrotoxic acute kidney injury in mice. Kidney Int 76:277–285
Mahmoud AM, Hussein OE, Hozayen WG, Abd el-Twab SM, (2017a) Methotrexate hepatotoxicity is associated with oxidative stress, and down-regulation of PPARγ and Nrf2: Protective effect of 18β-Glycyrrhetinic acid. Chem Biol Interact 270:59–72
Mahmoud AM, Mohammed HM, Khadrawy SM, Galaly SR (2017b) Hesperidin protects against chemically induced hepatocarcinogenesis via modulation of Nrf2/ARE/HO-1, PPARγ and TGF-β1/Smad3 signaling, and amelioration of oxidative stress and inflammation. Chem Biol Interact 277:146–158
Mahmoud AM, Hussein OE, Abd El-Twab SM, Hozayen WG (2019) Ferulic acid protects against methotrexate nephrotoxicity via activation of Nrf2/ARE/HO-1 signaling and PPARγ, and suppression of NF-κB/NLRP3 inflammasome axis. Food Funct 10:4593–4607
Maiorino M, Conrad M, Ursini F (2018) GPx4, lipid peroxidation, and cell death: discoveries, rediscoveries, and open issues. Antioxid Redox Signal 29:61–74
Neradil J, Pavlasova G, Veselská R (2012) New mechanisms for an old drug; DHFR-and non-DHFR-mediated effects of methotrexate in cancer cells. Klin Onkol 25:2S87-82S92
Oguz E, Kocarslan S, Tabur S, Sezen H, Yilmaz Z, Aksoy N (2015) Effects of lycopene alone or combined with melatonin on methotrexate-induced nephrotoxicity in rats. Asian Pac J Cancer Prev 16:6061–6066
Öktem F, Yilmaz HR, Ozguner F, Olgar S, Ayata A, Uzar E, Uz E (2006) Methotrexate-induced renal oxidative stress in rats: the role of a novel antioxidant caffeic acid phenethyl ester. Toxicol Ind Health 22:241–247
Pabla N, Dong Z (2008) Cisplatin nephrotoxicity: mechanisms and renoprotective strategies. Kidney Int 73:994–1007
Prince PS, Dhanasekar K, Rajakumar S (2011) Preventive effects of vanillic acid on lipids, bax, bcl-2 and myocardial infarct size on isoproterenol-induced myocardial infarcted rats: a biochemical and in vitro study. Cardiovasc Toxicol 11:58–66
Prince PSM, Dhanasekar K, Rajakumar S (2015) Vanillic acid prevents altered ion pumps, ions, inhibits Fas-receptor and caspase mediated apoptosis-signaling pathway and cardiomyocyte death in myocardial infarcted rats. Chem Biol Interact 232:68–76
Ramadan SS, Almeer R, Albasher G, Abdel Moneim AE (2022) Lycopene mitigates arsenic-induced nephrotoxicity with activation of the Nrf2 pathway in mice. Toxin Reviews 41:446–456
Ramsey LB, Balis FM, O’Brien MM, Schmiegelow K, Pauley JL, Bleyer A, Widemann BC, Askenazi D, Bergeron S, Shirali A (2018) Consensus guideline for use of glucarpidase in patients with high-dose methotrexate induced acute kidney injury and delayed methotrexate clearance. Oncologist 23:52–61
Shih AY, Li P, Murphy TH (2005) A small-molecule-inducible Nrf2-mediated antioxidant response provides effective prophylaxis against cerebral ischemia in vivo. Journal Neursci 25:10321–10335
Sindhu G, Nishanthi E, Sharmila R (2015) Nephroprotective effect of vanillic acid against cisplatin induced nephrotoxicity in wistar rats: a biochemical and molecular study. Environ Toxicol Pharmacol 39:392–404
Türk E, Güvenç M, Cellat M, Uyar A, Kuzu M, Ağgül AG, Kırbaş A (2022) Zingerone protects liver and kidney tissues by preventing oxidative stress, inflammation, and apoptosis in methotrexate-treated rats. Drug Chem Toxicol 45:1054–1065
Vishnu KV, Ajeesh Kumar KK, Chatterjee NS, Lekshmi RGK, Sreerekha PR, Mathew S, Ravishankar CN (2018) Sardine oil loaded vanillic acid grafted chitosan microparticles, a new functional food ingredient: attenuates myocardial oxidative stress and apoptosis in cardiomyoblast cell lines (H9c2). Cell Stress Chaperones 23:213–222
Widemann BC, Balis FM, Kempf-Bielack B, Bielack S, Pratt CB, Ferrari S, Bacci G, Craft AW, Adamson PC (2004) High-dose methotrexate-induced nephrotoxicity in patients with osteosarcoma: incidence, treatment, and outcome. Cancer 100:2222–2232
Yetiştirme DH, Erdem MG, Cinkılıç N, Vatan Ö, Yılmaz D, Bağdaş D, Bilaloğlu R (2012) Genotoxic and anti-genotoxic effects of vanillic acid against mitomycin C-induced genomic damage in human lymphocytes in vitro. Asian Pac J Cancer Prev 13:4993–4998
Yuksel Y, Yuksel R, Yagmurca M, Haltas H, Erdamar H, Toktas M, Ozcan O (2017) Effects of quercetin on methotrexate-induced nephrotoxicity in rats. Hum Exp Toxicol 36:51–61
Zoja C, Benigni A, Remuzzi G (2014) The Nrf2 pathway in the progression of renal disease. Nephrol Dial Transplant 29:i19–i24
Acknowledgements
This paper was extracted from an MD thesis written by Ms. Mahla Hasanzadeh Shoshtari, a student of medicine at Jundishapur University of Medical Sciences, Ahvaz, Iran. The authors would like to express their gratitude for the assistance of the Persian Gulf Physiology Research Center, Ahvaz Jundishapur University of Medical Sciences.
Funding
This work was supported financially by the Persian Gulf Physiology Research Center (APRC-0102) funded by the Vice Chancellor of Research, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran.
Author information
Authors and Affiliations
Contributions
NA designed the study and wrote the manuscript. MH contributed to the data collection. MB performed the data analysis and interpreted the results. FN performed molecular analysis and the histology. Mahin Dianat contributed to the data analysis. All authors read and approved the final version of the manuscript.The authors declare that all data were generated in-house and that no paper mill was used.
Corresponding author
Ethics declarations
Ethical considerations
The current research was confirmed by the Animal Ethics Committee of Ahvaz Jundishapur University of Medical Sciences, Iran (IR.AJUMS.ABHC.REC.1401.009).
Consent to participate
This is an animal study.
Consent for publication
All authors agree to publish.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Amini, N., Shoshtari, M.H., Nejaddehbashi, F. et al. Dose-dependent renoprotective effect of vanillic acid on methotrexate-induced nephrotoxicity via its anti-apoptosis, antioxidant, and anti-inflammatory properties. Naunyn-Schmiedeberg's Arch Pharmacol (2023). https://doi.org/10.1007/s00210-023-02866-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00210-023-02866-y