Abstract
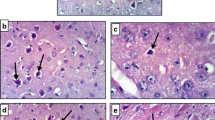
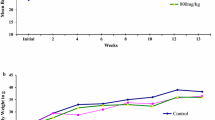
The study was undertaken to evaluate the safety of vitacamphorae (VCP) injection in Sprague-Dawley (SD) rats. Rats were intravenously administered with VCP at the doses of 0, 5, 15, and 50 mg/kg/day (equivalent to 0, 5, 15, and 50 times the clinical equivalent dose) for 4 weeks, respectively. In addition, we also tested oxidative stress-related parameters and cytokine levels in rat serum. In the current study, intravenous administration of VCP at a dose of 50 mg/kg/day caused significant pathophysiological responses in rats. Compared with the control group, different doses of VCP exposure had no significant effect on body weight, food consumption, and clinic pathology of rats after 4 weeks of VCP administration. Rats in high-dose group (50 mg/kg/day) showed general symptoms of convulsions after VCP administration. The toxicological significance of VCP exposure in the spleen of high-dose female rats was observed, which showed a significant increase in the relative spleen weights (P < 0.01) and mild lymphocyte proliferation in splenic pathology. Furthermore, the results of oxidative stress and cytokine detection showed that the levels of antioxidant enzymes SOD increased in each administration group, but the levels of a series of pro-inflammatory cytokines IL-1β, IL-6, IL-8, IL-12, and IFN-γ also increased in these groups. Above changes caused by VCP exposure can be reversed after 4 weeks of recovery. Overall, the results showed that the no-observed-adverse-effect-level (NOAEL) of VCP injection for 4-week toxicity was 15 mg/kg/day.




Similar content being viewed by others
References
Chen Y, Chen K, Jin Q (2015) Influence of rosuvastatin on blood lipid, inflammatory factor and oxidative stress index of patients with chronic heart failure [J]. J Hainan Med Univ 9:15–18
Deng H, Yang H (2018) Effects of Yupingfeng polysaccharides on the spleen tissue structure of acute and chronic Immunocompromised mice [J]. Medicinal Plant 5:93–96
Fang J, Tang Y, Cheng X, Wang L, Cai C, Zhang X, Liu S, Li P (2019) Exenatide alleviates adriamycin-induced heart dysfunction in mice: modulation of oxidative stress, apoptosis and inflammation [J]. Chem Biol Interact 304:186–193
Farías JG, Herrera EA, Carrasco-Pozo et al (2016) Pharmacological models and approaches for pathophysiological conditions associated with hypoxia and oxidative stress [J]. Pharmacol Ther 158:1–23
Guo Y, Di T, Zhao J et al (2018) Fuxin decoction attenuates doxorubicin-induced heart failure in rats via oxidizing suppression and regulating immune responses [J]. J Tradit Chin Med 38(04):579–584
Hedayat M, Mahmoudi MJ, Rose NR et al (2010) Pro-inflammatory cytokines in heart failure: double-edged swords [J]. Heart Fail Rev 15(6):543–562
Inagi R (2006) Oxidative Stress in Cardiovascular Disease: A New Avenue Toward Future Therapeutic Approaches [J]. Recent Pat Cardiovasc Drug Discov 1(2)
Jiang S, Li T, Du P, et al. Synthesis of vitacamphor derivatives of camphor and its preliminary anti-inflammatory activity [C]. International Conference on Human Health and Biomedical Engineering, IEEE, 2011
Liang C, Yang Z, Meng X et al (2016) Development of a GC-MS method for the determination and pharmacokinetics of trans-π-oxocamphor after intravenous administration of Vitacamphorae injection in rat [J]. Anal Methods 8:4720–4726
Lin J, Pang L, Liu XL et al (2016) Role of vitacamphore in improving central pro-inflammatory cytokines following transient global ischemia [J]. J Biol Regul Homeost Agents 30(4):1091–1098
Liu M, Chen J, Huang D, Ke J, Wu W (2014) A meta-analysis of pro-inflammatory cytokines in chronic heart failure [J]. Heart Asia 6(1):130–136
Miyazawa M, Hashimoto Y, Taniguchi Y, Kubota K (2001) Headspace constituents of the tree remain of Cinnamomum camphora [J]. Nat Prod Lett 15(1):63–69
Muslum K, Mehmet KF, Serkan Y et al (2018) Morin attenuates doxorubicin-induced heart and brain damage by reducing oxidative stress, inflammation and apoptosis [J]. Biomed Pharmacother 106:443–453
Ren Y, Chen X, Li P et al (2019) Si-Miao-Yong-an decoction ameliorates cardiac function through restoring the equilibrium of SOD and NOX2 in heart failure mice [J]. Pharmacol Res 146:104318
Romuk E, Jacheć W, Skrzep-Poloczek B et al (2011) The assessment of lipid peroxidation products and the activity of superoxide dismutase in patients with heart failure [J]. Wiadomości Lekarskie 64(2):75
Samson J, Devi RS, Ravindran R et al (2005) Effect of noise stress on free radical scavenging enzymes in brain [J]. Environ Toxicol Pharmacol 20(1):142–148
Somade OT, Adeniji KD, Adesina ARA et al (2017) Oral acute toxicity study as well as tissues oxidative stress and histopathological disorders in edible camphor administered rats [J]. Exp Toxicol Pathol 69(2):99–108
Tsutsui H, Kinugawa S, Matsushima S (2011) Oxidative stress and heart failure [J]. Am J Phys Heart Circ Phys 301(6):H2181–H2190
Acknowledgments
This work was supported by Jiangsu Center for Safety Evaluation of Drugs. Thanks to Ms. Zhou for her guidance on this work and writing.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
Y Y, M S, Y L, J L, Y X, and B Z were involved in the design and performance of the experiments. Y Y, M S, and B Z drafted and revised the manuscript. All authors approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The study related to animal was approved by Institutional Animal Care and Use Committee (IACUC) in Jiangsu center for safety evaluation of drugs. All operations are in compliance with ethical requirements and aiming to minimize the number of rats used and their suffering.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yuan, Y., Su, M., Liu, J. et al. Four-week intravenous repeated dose toxicity study of vitacamphorae injection in rats. Naunyn-Schmiedeberg's Arch Pharmacol 393, 2001–2007 (2020). https://doi.org/10.1007/s00210-020-01820-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-020-01820-6