Abstract
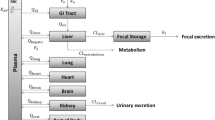
As a toxic substance, 4-n-nonylphenol (4-n-NP) or 4-nonylphenol (4-NP) is widely present in the environment. 4-n-NP is a single substance with a linear-alkyl side chain, but 4-NP usually refers to a random mixture containing various branched types. Unfortunately, human risk assessment and/or exposure level analysis for 4-n-NP (or 4-NP) were almost nonexistent, and related research was urgently needed. This study aimed to analyze the various exposures of 4-n-NP (or 4-NP) through development of a physiologically based-pharmacokinetic (PBPK) model considering gender difference in pharmacokinetics of 4-n-NP and its application to human risk assessment studies. A PBPK model was newly developed considering gender differences in 4-n-NP pharmacokinetics and applied to a human risk assessment for each gender. Exposure analysis was performed using a PBPK model that considered gender differences in 4-n-NP (or 4-NP) exposure and high variabilities in several countries. Furthermore, an extended application was attempted as a human risk assessment for random mixture 4-NP, which is difficult to accurately evaluate in reality. External-exposure and margin-of-safety estimated with the same internal exposure amount differed between genders, meaning the need for a differentiated risk assessment considering gender. Exposure analysis based on biomonitoring data confirmed large variability in exposure to 4-n-NP (or 4-NP) by country, group, and period. External-exposures estimated using PBPK model varied widely, ranging from 0.039 to 63.875 mg/kg/day (for 4-n-NP or 4-NP). By country, 4-n-NP (or 4-NP) exposure was higher in females than in males and the margin-of-safety tended to be low. Overall, exposure to 4-n-NP (or 4-NP) in populations was largely not safe, suggesting need for ongoing management and monitoring. Considering low in vivo accumulation confirmed by PBPK model, risk reduction of 4-n-NP is possible by reducing its use.











Similar content being viewed by others
Abbreviations
- 4-n-NP:
-
4-n-Nonylphenol
- 4-NP:
-
4-Nonylphenol
- PBPK:
-
Physiologically based pharmacokinetic
- UHPLC-ESI-MS/MS:
-
Ultrahigh performance liquid chromatography electrospray ionization mass spectrometry
- GI:
-
Gastrointestinal
- ESI:
-
Electrospray ionization
- RMSE:
-
Root mean squared error
- AIC:
-
Akaike’s information criterion
- CI:
-
Confidence interval
- ANOVA:
-
Analysis of variance
- NOAEL:
-
No observed adverse effect level
- POD:
-
Point of departure
- CV:
-
Coefficient of variation
References
Ademollo N, Ferrara F, Delise M, Fabietti F, Funari E (2008) Nonylphenol and octylphenol in human breast milk. Environ Int 34:984–987. https://doi.org/10.1016/j.envint.2008.03.001
Asimakopoulos AG, Thomaidis NS, Koupparis MA (2012) Recent trends in biomonitoring of bisphenol A, 4-t-octylphenol, and 4-nonylphenol. Toxicol Lett 210:141–154. https://doi.org/10.1016/j.toxlet.2011.07.032
Azzouz A, Rascón AJ, Ballesteros E (2016) Simultaneous determination of parabens, alkylphenols, phenylphenols, bisphenol A and triclosan in human urine, blood and breast milk by continuous solid-phase extraction and gas chromatography–mass spectrometry. J Pharm Biomed Anal 119:16–26. https://doi.org/10.1016/j.jpba.2015.11.024
Babich H, Davis D (1981) Phenol: a review of environmental and health risks. Regul Toxicol Pharm 1:90–109
Bethea TN, Wesselink AK, Weuve J et al (2020) Correlates of exposure to phenols, parabens, and triclocarban in the study of environment, lifestyle and fibroids. J Expo Sci Environ Epidemiol 30:117–136. https://doi.org/10.1038/s41370-019-0114-9
Bruce RM, Santodonato J, Neal MW (1987) Summary review of the health effects associated with phenol. Toxicol Ind Health 3:535–568
Calafat AM, Kuklenyik Z, Reidy JA, Caudill SP, Ekong J, Needham LL (2005) Urinary concentrations of bisphenol A and 4-nonylphenol in a human reference population. Environ Health Perspect 113:391–395
Cao Y, Jusko WJ (2012) Applications of minimal physiologically-based pharmacokinetic models. J Pharmacokinet Pharmacodyn 39:711–723. https://doi.org/10.1007/s10928-012-9280-2
Chang C-H, Chen M-L, Liao K-W et al (2013) The association between maternal nonylphenol exposure and parity on neonatal birth weight: a cohort study in Taiwan. Chemosphere 93:1145–1152. https://doi.org/10.1016/j.chemosphere.2013.06.048
Chen M-L, Lee W-P, Chung H-Y, Guo B-R, Mao I-F (2005) Biomonitoring of alkylphenols exposure for textile and housekeeping workers. Int J Environ Anal Chem 85:335–347
Chen M-L, Lee H-Y, Chuang H-Y, Guo B-R, Mao IF (2009) Association between nonylphenol exposure and development of secondary sexual characteristics. Chemosphere 76:927–931. https://doi.org/10.1016/j.chemosphere.2009.04.054
Chen G-W, Ding W-H, Ku H-Y et al (2010) Alkylphenols in human milk and their relations to dietary habits in central Taiwan. Food Chem Toxicol 48:1939–1944. https://doi.org/10.1016/j.fct.2010.04.038
Chen M, Tang R, Fu G et al (2013) Association of exposure to phenols and idiopathic male infertility. J Hazard Mater 250–251:115–121. https://doi.org/10.1016/j.jhazmat.2013.01.061
Choi H, Kim J, Im Y, Lee S, Kim Y (2012) The association between some endocrine disruptors and hypospadias in biological samples. J Environ Sci Health A Tox Hazard Subst Environ Eng 47:2173–2179. https://doi.org/10.1080/10934529.2012.680387
Choi J, Eom J, Kim J, Lee S, Kim Y (2014) Association between some endocrine-disrupting chemicals and childhood obesity in biological samples of young girls: a cross-sectional study. Environ Toxicol Pharm 38:51–57. https://doi.org/10.1016/j.etap.2014.04.004
Choi G-W, Lee Y-B, Cho H-Y (2019) Interpretation of non-clinical data for prediction of human pharmacokinetic parameters: in vitro-in vivo extrapolation and allometric scaling. Pharmaceutics 11:168. https://doi.org/10.3390/pharmaceutics11040168
Chung S-H, Ding W-H (2018) Isotope-dilution gas chromatography-mass spectrometry coupled with injection-port butylation for the determination of 4-t-octylphenol, 4-nonylphenols and bisphenol A in human urine. J Pharm Biomed Anal 149:572–576. https://doi.org/10.1016/j.jpba.2017.11.063
Cunny H, Mayes B, Rosica K, Trutter J, Van Miller J (1997) Subchronic toxicity (90-day) study withpara-nonylphenol in rats. Regul Toxicol Pharm 26:172–178
Davies B, Morris T (1993) Physiological parameters in laboratory animals and humans. Pharm Res 10:1093–1095
ECB (2002) European union risk assessment report: 4-nonylphenol (branched) and nonylphenol. European chemicals bureau, Joint Research Centre, European Commission, Ispra, Italy
EPA (2006) Approaches for the application of physiologically based pharmacokinetic (PBPK) models and supporting data in risk assessment (final report). US Environmental Protection Agency, Washington, DC, USA
Fan Z, Shi H, Zhao H, Cai J, Zhao G (2018) Application of carbon aerogel electrosorption for enhanced Bi2WO6 photoelectrocatalysis and elimination of trace nonylphenol. Carbon 126:279–288. https://doi.org/10.1016/j.carbon.2017.10.009
Gautam GJ, Chaube R, Joy K (2015) Toxicity and tissue accumulation of 4-nonylphenol in the catfish Heteropneustes fossilis with a note on prevalence of 4-NP in water samples. Endocr Disruptors. https://doi.org/10.4161/23273747.2014.981442
Helkar PB, Sahoo A, Patil N (2016) Review: food industry by-products used as a functional food ingredients. Int J Waste Resour 6:1–6
Huang Y-F, Pan W-C, Tsai Y-A et al (2017) Concurrent exposures to nonylphenol, bisphenol A, phthalates, and organophosphate pesticides on birth outcomes: a cohort study in Taipei. Taiwan Sci Total Environ 607–608:1126–1135. https://doi.org/10.1016/j.scitotenv.2017.07.092
Igari Y, Sugiyama Y, Sawada Y, Iga T, Hanano M (1983) Prediction of diazepam disposition in the rat and man by a physiologically based pharmacokinetic model. J Pharmacokinet Biopharm 11:577–593
Inoue K, Kawaguchi M, Okada F et al (2003) Measurement of 4-nonylphenol and 4-tert-octylphenol in human urine by column-switching liquid chromatography–mass spectrometry. Anal Chim Acta 486:41–50. https://doi.org/10.1016/S0003-2670(03)00464-1
Jeong S-H, Jang J-H, Cho H-Y, Lee Y-B (2019) Gender differences in pharmacokinetics and tissue distribution of 4-n-nonylphenol in rats. Arch Toxicol 93:3121–3139. https://doi.org/10.1007/s00204-019-02581-9
Jeong S-H, Jang J-H, Cho H-Y, Lee Y-B (2020) Risk assessment for humans using physiologically based pharmacokinetic model of diethyl phthalate and its major metabolite, monoethyl phthalate. Arch Toxicol 94:2377–2400. https://doi.org/10.1007/s00204-020-02748-9
Jeong S-H, Jang J-H, Cho H-Y, Lee Y-B (2021) Human risk assessment of di-isobutyl phthalate through the application of a developed physiologically based pharmacokinetic model of di-isobutyl phthalate and its major metabolite mono-isobutyl phthalate. Arch Toxicol 95:2385–2402. https://doi.org/10.1007/s00204-021-03057-5
Ji X, Li N, Yuan S et al (2019) A comparison of endocrine disruption potential of nonylphenol ethoxylate, vanillin ethoxylate, 4-n-nonylphenol and vanillin in vitro. Ecotoxicol Environ Saf 175:208–214. https://doi.org/10.1016/j.ecoenv.2019.03.060
Kabir ER, Rahman MS, Rahman I (2015) A review on endocrine disruptors and their possible impacts on human health. Environ Toxicol Pharm 40:241–258. https://doi.org/10.1016/j.etap.2015.06.009
Kassotis CD, Vandenberg LN, Demeneix BA, Porta M, Slama R, Trasande L (2020) Endocrine-disrupting chemicals: economic, regulatory, and policy implications. Lancet Diabetes Endocrinol 8:719–730. https://doi.org/10.1016/S2213-8587(20)30128-5
Kawaguchi M, Inoue K, Sakui N et al (2004) Stir bar sorptive extraction and thermal desorption–gas chromatography–mass spectrometry for the measurement of 4-nonylphenol and 4-tert-octylphenol in human biological samples. J Chromatogr B 799:119–125. https://doi.org/10.1016/j.jchromb.2003.10.021
Kawaguchi M, Sakui N, Okanouchi N et al (2005) Stir bar sorptive extraction with in situ derivatization and thermal desorption-gas chromatography–mass spectrometry for measurement of phenolic xenoestrogens in human urine samples. J Chromatogr B 820:49–57. https://doi.org/10.1016/j.jchromb.2005.03.019
Kawaguchi M, Ito R, Sakui N et al (2007) Stir-bar-sorptive extraction, with in-situ deconjugation, and thermal desorption with in-tube silylation, followed by gas chromatography-mass spectrometry for measurement of urinary 4-nonylphenol and 4-tert-octylphenol glucuronides. Anal Bioanal Chem 388:391–398. https://doi.org/10.1007/s00216-007-1225-z
Kenyon E (2012) Interspecies extrapolation. In: Reisfeld B, Mayeno AN (eds) Computational toxicology: methods in molecular biology. Humana Press, Totowa, NJ, USA, pp 501–520
Kim S-J, Shin H, Lee Y-B, Cho H-Y (2018) Sex-specific risk assessment of PFHxS using a physiologically based pharmacokinetic model. Arch Toxicol 92:1113–1131. https://doi.org/10.1007/s00204-017-2116-5
Kim S-J, Choi E-J, Choi G-W, Lee Y-B, Cho H-Y (2019) Exploring sex differences in human health risk assessment for PFNA and PFDA using a PBPK model. Arch Toxicol 93:311–330. https://doi.org/10.1007/s00204-018-2365-y
Kuklenyik Z, Ekong J, Cutchins CD, Needham LL, Calafat AM (2003) Simultaneous measurement of urinary bisphenol A and alkylphenols by automated solid-phase extractive derivatization gas chromatography/mass spectrometry. Anal Chem 75:6820–6825. https://doi.org/10.1021/ac0303158
Li X, Ying G-G, Zhao J-L, Chen Z-F, Lai H-J, Su H-C (2013) 4-nonylphenol, bisphenol-A and triclosan levels in human urine of children and students in China, and the effects of drinking these bottled materials on the levels. Environ Int 52:81–86. https://doi.org/10.1016/j.envint.2011.03.026
Lin W-C, Wang S-L, Cheng C-Y, Ding W-H (2009) Determination of alkylphenol residues in breast and commercial milk by solid-phase extraction and gas chromatography–mass spectrometry. Food Chem 114:753–757. https://doi.org/10.1016/j.foodchem.2008.10.059
Liu T, Cao W, Di Q, Zhao M, Xu Q (2019) Evaluation of toxicokinetics of nonylphenol in the adult female sprague-dawley rats using a physiologically based toxicokinetic model. Regul Toxicol Pharmacol 105:42–50. https://doi.org/10.1016/j.yrtph.2019.03.019
Lu D, Yu L, Li M, Zhai Q, Tian F, Chen W (2021) Behavioral disorders caused by nonylphenol and strategies for protection. Chemosphere 275:129973. https://doi.org/10.1016/j.chemosphere.2021.129973
Maguire RJ (1999) Review of the persistence of nonylphenol and nonylphenol ethoxylates in aquatic environments. Water Qual Res J 34:37–78
Naidu R, Biswas B, Willett IR et al (2021) Chemical pollution: a growing peril and potential catastrophic risk to humanity. Environ Int. https://doi.org/10.1016/j.envint.2021.106616
NICNAS (2019) Nonylphenols: human health tier II assessment IMAP group assessment report. National Industrial Chemicals Notification and Assessments Scheme, Australian Government, Sydney, NSW, Australia
Osimitz TG, Droege W, Driver JH (2015) Human risk assessment for nonylphenol. Hum Ecol Risk Assess 21:1903–1919. https://doi.org/10.1080/10807039.2014.999520
Otaka H, Yasuhara A, Morita M (2003) Determination of bisphenol A and 4-nonylphenol in human milk using alkaline digestion and cleanup by solid-phase extraction. Anal Sci 19:1663–1666. https://doi.org/10.2116/analsci.19.1663
Park H, Kim K (2017) Urinary levels of 4-nonylphenol and 4-t-octylphenol in a representative sample of the Korean adult population. Int J Environ Res Public Health 14:932. https://doi.org/10.3390/ijerph14080932
Peng F, Ji W, Zhu F et al (2016) A study on phthalate metabolites, bisphenol A and nonylphenol in the urine of Chinese women with unexplained recurrent spontaneous abortion. Environ Res 150:622–628. https://doi.org/10.1016/j.envres.2016.04.003
Pirard C, Sagot C, Deville M, Dubois N, Charlier C (2012) Urinary levels of bisphenol A, triclosan and 4-nonylphenol in a general Belgian population. Environ Int 48:78–83. https://doi.org/10.1016/j.envint.2012.07.003
Price PS, Conolly RB, Chaisson CF et al (2003) Modeling interindividual variation in physiological factors used in PBPK models of humans. Crit Rev Toxicol 33:469–503
Ringbeck B, Belov VN, Schmidtkunz C et al (2021) Human metabolism and urinary excretion kinetics of nonylphenol in three volunteers after a single oral dose. Chem Res Toxicol 34:2392–2403. https://doi.org/10.1021/acs.chemrestox.1c00301
Soares A, Guieysse B, Jefferson B, Cartmell E, Lester J (2008) Nonylphenol in the environment: a critical review on occurrence, fate, toxicity and treatment in wastewaters. Environ Int 34:1033–1049. https://doi.org/10.1016/j.envint.2008.01.004
Tang R, Chen M-j, Ding G-d et al (2013) Associations of prenatal exposure to phenols with birth outcomes. Environ Pollut 178:115–120. https://doi.org/10.1016/j.envpol.2013.03.023
Vargas-Berrones K, Bernal-Jácome L, de León-Martínez LD, Flores-Ramírez R (2020) Emerging pollutants (EPs) in Latin América: a critical review of under-studied EPs, case of study-nonylphenol. Sci Total Environ 726:138493. https://doi.org/10.1016/j.scitotenv.2020.138493
Verner M-A, Longnecker MP (2015) Comment on “enhanced elimination of perfluorooctanesulfonic acid by menstruating women: evidence from population-based pharmacokinetic modeling.” Environ Sci Technol 49:5836–5837. https://doi.org/10.1021/acs.est.5b00187
Wang H, Yan H, Wang C et al (2012) Analysis of phenolic pollutants in human samples by high performance capillary electrophoresis based on pretreatment of ultrasound-assisted emulsification microextraction and solidification of floating organic droplet. J Chromatogr A 1253:16–21. https://doi.org/10.1016/j.chroma.2012.06.088
Wang P-W, Chen M-L, Huang L-W, Yang W, Wu K-Y, Huang Y-F (2015) Prenatal nonylphenol exposure, oxidative and nitrative stress, and birth outcomes: a cohort study in Taiwan. Environ Pollut 207:145–151. https://doi.org/10.1016/j.envpol.2015.08.044
Wang Z, Walker GW, Muir DC, Nagatani-Yoshida K (2020) Toward a global understanding of chemical pollution: a first comprehensive analysis of national and regional chemical inventories. Environ Sci Technol 54:2575–2584. https://doi.org/10.1021/acs.est.9b06379
WHO (2010) Characterization and application of physiologically based pharmacokinetic models in risk assessment. International programme on chemical safety, World Health Organization, Geneva, Switzerland
Wilson NK, Chuang JC, Morgan MK, Lordo RA, Sheldon LS (2007) An observational study of the potential exposures of preschool children to pentachlorophenol, bisphenol-A, and nonylphenol at home and daycare. Environ Res 103:9–20. https://doi.org/10.1016/j.envres.2006.04.006
Wong F, MacLeod M, Mueller JF, Cousins IT (2014) Enhanced elimination of perfluorooctane sulfonic acid by menstruating women: evidence from population-based pharmacokinetic modeling. Environ Sci Technol 48:8807–8814. https://doi.org/10.1021/es500796y
Yi B-N, Kim C-S, Park M-J, Han Y-S, Lee S-J, Yan M-H (2008) Exposure monitoring of nonylphenol in preterm breast milk in seoulers. Environ Health Toxicol 23:113–117
Ying G-G, Williams B, Kookana R (2002) Environmental fate of alkylphenols and alkylphenol ethoxylates-a review. Environ Int 28:215–226
Zhou F, Zhang L, Liu A et al (2013) Measurement of phenolic environmental estrogens in human urine samples by HPLC–MS/MS and primary discussion the possible linkage with uterine leiomyoma. J Chromatogr B 938:80–85. https://doi.org/10.1016/j.jchromb.2013.08.032
Acknowledgements
This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI) funded by the Ministry of Health and Welfare, Republic of Korea (No. HI21C1032). This research was also supported by a grant of the Basic Science Research Program through the National Research Foundation (NRF) funded by the Ministry of Education, Republic of Korea (No. 2021R1A6A3A03039851).
Author information
Authors and Affiliations
Contributions
S-HJ conceptualization, investigation, methodology, writing—original draft, writing—review and editing, software, data analysis, visualization; J-HJ conceptualization, investigation, writing—review and editing, software, data analysis; H-YC writing—review, project administration, conceptualization; YB L conceptualization, methodology, writing—review and editing, funding acquisition, supervision.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interests regarding the publication of this paper.
Ethical approval
All animal experiments were approved by Chonnam National University Animal Experimental Ethics Committee (No. CNU IACUC-YB-2020-60 and CNU IACUC-YB-2017-45), Republic of Korea. This study was performed according to revised Guidelines for Ethical Conduct in the Care and Use of Animals and rules of Good Laboratory Practice.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Jeong, SH., Jang, JH., Cho, HY. et al. Human risk assessment of 4-n-nonylphenol (4-n-NP) using physiologically based pharmacokinetic (PBPK) modeling: analysis of gender exposure differences and application to exposure analysis related to large exposure variability in population. Arch Toxicol 96, 2687–2715 (2022). https://doi.org/10.1007/s00204-022-03328-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-022-03328-9